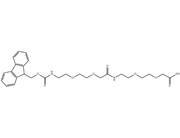
Synonyms: | Fmoc-L-Lys[Oct-(otBu)-Glu-(otBu)-AEEA-AEEA]-OH;Octa(OtBu)-Glu(ɑ-OtBu)-AEEA-AEEA-OH;11,14,20,23-Tetraoxa-2,8,17,26,31-pentaazanonatetracontanedioicacid,3-carboxy-30-[(1,1-dimethylethoxy)carbonyl]-9,18,27,32-tetraoxo-,49-(1,1-dimethylethyl)1-(9H-fluoren-9-ylmethyl)ester,;22-(tert-butoxycarbonyl)-43,43-dimethyl-10,19,24,41-tetraoxo-3,6,12,15,42-pentaoxa-9,18,23-triazatetratetracontanoicacid;Fmoc-Lys(tBu-OOC-C16-CO-Glu(AEEA-AEEA)-OtBu)-OH;11,14,20,23-Tetraoxa-2,8,17,26,31-pentaazanonatetracontanedioicacid,3-carboxy-30-[(1,1-dimethylethoxy)carbonyl]-9,18,27,32-tetraoxo-,49-(1,1-dimethylethyl)1-(9H-fluoren-9-ylmethyl)ester,(3S,30S)-;Fmoc-Lys-(Octa-OtBu)-Glu(AEEA-AEEA-OH)-OH;(23S,50S)-50-((((9H-Fluoren-9-yl)methoxy)carbonyl)amino)-23-(tert-butoxycarbonyl)-2,2-dimethyl-4,21,26,35,44-pentaoxo-3,30,33,39,42-pentaoxa-22,27,36,45-tetraazahenpentacontan-51-oicacid |
Application: |
The specific application of Fmoc-L-Lys[Oct-(otBu)-Glu-(otBu)-AEEA-AEEA]-OH is typically in peptide synthesis, specifically as a building block or intermediate in the solid-phase peptide synthesis (SPPS) of longer peptides or polypeptides. This compound belongs to a class of modified amino acids that are chemically modified to improve their stability and solubility during peptide synthesis.
The Fmoc (fluorenylmethoxycarbonyl) group is a widely used amino-protecting group in SPPS. It is stable under basic conditions, allowing for the selective deprotection of the amino group for subsequent coupling reactions. The Fmoc group is removed under acidic conditions, facilitating the attachment of the next amino acid residue.
The specific structure of Fmoc-L-Lys[Oct-(otBu)-Glu-(otBu)-AEEA-AEEA]-OH suggests that it is designed for the synthesis of peptides containing lysine as the N-terminal residue, followed by a series of amino acid modifications. The "Oct-(otBu)" modification likely refers to an octahydroxylated derivative of lysine, which may enhance the solubility or stability of the peptide. The "Glu-(otBu)" and "AEEA" (2-aminoethylethanolamine) moieties introduce additional chemical functionality or modulation of the peptide's biological properties.
The specific application of this compound depends on the research goals and the desired properties of the final peptide. It could be used in the synthesis of peptides for biological studies, drug discovery, or the development of novel therapeutic agents. The modified amino acids provide researchers with tools to fine-tune the peptide's physical, chemical, and biological properties to achieve desired therapeutic effects or research outcomes. |
![Fmoc-L-Lys[Oct-(otBu)-Glu-(otBu)-AEEA-AEEA]-OH](/ProductImageEN/2024-03/Large/5788cfee-62b6-42bf-a6ae-b61613967a8e.gif)

 China
China