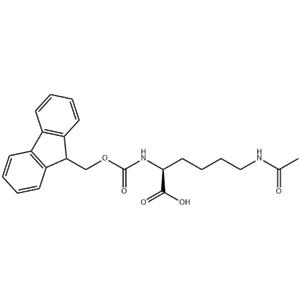
Peptide Synthesis: As a building block in peptide synthesis, Fmoc-L-Lys(AC) allows for the selective addition of lysine residues to the growing peptide chain. The acetyl group protects the amine group during the coupling process, preventing unwanted side reactions. Once the peptide is synthesized, the acetyl group can be removed to reveal the free amine, which can then be further modified or conjugated if desired.
Selective Deprotection: The acetyl protecting group on Fmoc-L-Lys(AC) is relatively stable and can be removed selectively using mild conditions. This allows for the controlled deprotection of specific lysine residues in complex peptides, enabling subsequent modification or conjugation reactions. Bioconjugation: The free amine group of deprotected Fmoc-L-Lys(AC) can be used for bioconjugation reactions, such as attaching peptides to surfaces, carriers, or other biomolecules. This amine reactivity enables the immobilization of peptides for biosensor development, drug delivery systems, or tissue engineering applications. Peptide Modifications: The acetyl group on Fmoc-L-Lys(AC) can also be exploited for peptide modifications. For example, the acetyl group can be replaced with other functional groups or probes to introduce site-specific modifications that enhance the biological activity, stability, or detection of the peptide. | 
 China
China