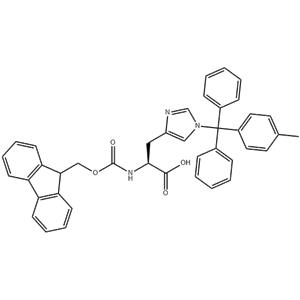
Solid-Phase Peptide Synthesis (SPPS):Fmoc-His(Mtt) is an essential building block in solid-phase peptide synthesis, especially when synthesizing peptides containing histidine residues. The Fmoc group allows for selective deprotection of the amino terminus using base, enabling coupling with other amino acids.
The Mtt group protects the histidine side chain from unwanted reactions during peptide synthesis. This protection is necessary because the imidazole group of histidine is prone to various side reactions that can disrupt the peptide synthesis process. Biological Assays and Protein-Ligand Interactions:Peptides containing Fmoc-His(Mtt) can be used in biological assays to study protein-ligand interactions. The histidine residue, once deprotected, can participate in metal ion coordination, hydrogen bonding, or other types of interactions with protein binding sites. These peptides can be labeled with fluorescent probes or other detection reagents to monitor binding events. They are useful in elucidating binding mechanisms or affinities of ligands for specific protein targets. Bioconjugation and Materials Science:The histidine residue in Fmoc-His(Mtt) can be used as a reactive handle for covalent attachment to other molecules or materials after deprotection. This bioconjugation finds applications in surface modification of biomaterials, immobilization of enzymes or antibodies, or the preparation of hydrogels and other biocompatible materials. The histidine residue can also be used for metal chelation, allowing the peptide conjugate to bind metal ions. This property can be exploited in various applications, such as targeted drug delivery or sensing and imaging. | 
 China
China