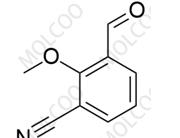
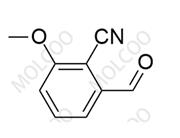
Finerenone Impurity 125
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Number: F044125
English Name: Finerenone Impurity 125
English Alias: 2-formyl-4-methoxybenzonitrile
CAS Number: 21962-52-7
Molecular Formula: C₉H₇NO₂
Molecular Weight: 161.16
As an impurity of Finerenone, this compound has the following advantages:
Well-defined and distinct structure: Contains formyl (-CHO), methoxy (-OCH₃), and benzonitrile (-CN) groups, with a para-substituted methoxy relative to the formyl group on the benzene ring. This structural feature distinguishes it from target intermediates of Finerenone, allowing accurate identification via HPLC, GC-MS, etc., as a specific marker for impurity detection;
High stability and traceability: The conjugated system of formyl, methoxy, and cyano groups is stable under neutral conditions. As a positional isomer by-product from benzene ring substitution reactions in Finerenone synthesis, it directly reflects the regioselectivity of the reaction, improving the accuracy of process tracing;
High detection sensitivity: The aldehyde group of formyl, conjugated with the benzene ring, shows characteristic UV absorption (270-290nm), and the electron-donating methoxy group enhances this absorption, enabling trace analysis via HPLC-UV, compatible with detection systems for aromatic aldehydes.
Pharmaceutical quality control: Used as an impurity reference standard to identify and quantify Finerenone Impurity 125 in Finerenone APIs and formulations, ensuring residual positional isomer impurities meet quality standards;
Synthesis process optimization: Optimizing reaction conditions (e.g., solvent, catalyst type) for benzene ring formylation and cyanation by monitoring impurity content, to enhance selectivity for the target ortho-methoxy isomer;
Intermediate purity assessment: Evaluating the purity of key benzene ring intermediates in Finerenone synthesis to support the specificity of subsequent cyclization reactions.
Finerenone, a non-steroidal mineralocorticoid receptor antagonist for type 2 diabetes-related chronic kidney disease, features a benzene ring with a specific substitution pattern. During synthesis, formylation and cyanation of the benzene ring may produce 2-formyl-4-methoxybenzonitrile (para-methoxy substitution) as a positional isomer impurity due to insufficient regioselectivity, instead of the target ortho-methoxy intermediate. The presence of this impurity can interfere with the specificity of subsequent ring-closure reactions, making its control critical for Finerenone quality assurance.
Current research focuses on:
Detection method advancement: Using UPLC-DAD with optimized parameters at 280nm (characteristic absorption of formyl-benzene conjugation) to achieve trace detection (ppm level);
Regioselectivity improvement: Developing sterically hindered catalysts or directed substitution reagents to suppress para-methoxy impurity formation by regulating electronic and steric effects on the benzene ring;
Reaction mechanism studies: Tracking reaction progress via ¹H NMR and DFT calculations to clarify the formation mechanism of positional isomers based on substituent effects;
Stability evaluations: Investigating impurity degradation under varying pH and temperature to guide storage conditions for Finerenone raw materials and formulations.
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com





 China
China