| CAS: | 7758-94-3 |
| MF: | Cl2Fe |
| MW: | 126.75 |
| EINECS: | 231-843-4 |
| Product Categories: | 26: Fe;Beaded Materials;Crystal Grade Inorganics;Iron;Iron Salts;Materials Science;Inorganics;metal halide;Metal and Ceramic Science;Salts;Catalysis and Inorganic Chemistry;Chemical Synthesis;7758-94-3 |
| Mol File: | 7758-94-3.mol |
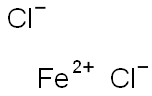 |
|
| Ferrous chloride Chemical Properties |
| Melting point | 677 °C (lit.) |
| Boiling point | 1023°C |
| density | 3.16 g/mL at 25 °C (lit.) |
| vapor pressure | 0Pa at 20℃ |
| Fp | 1023°C |
| solubility | H2O: soluble |
| form | beads |
| Specific Gravity | 3.162 |
| color | Off-white |
| Water Solubility | Soluble in water, alcohol and acetone. Slightly soluble in benzene. Insoluble in ether. |
| Sensitive | Hygroscopic |
| Merck | 14,4043 |
| Exposure limits | ACGIH: TWA 1 mg/m3
NIOSH: TWA 1 mg/m3 |
| InChIKey | NMCUIPGRVMDVDB-UHFFFAOYSA-L |
| CAS DataBase Reference | 7758-94-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Iron dichloride(7758-94-3) |
| EPA Substance Registry System | Ferrous chloride (7758-94-3) |
| Ferrous chloride Usage And Synthesis |
| Occurrence and Uses | Iron(II) chloride occurs in nature as the mineral lawrencite. Iron dichloride is used as a mordant for dyeing; and as a reducing agent. It also is used in pharmaceutical preparation; in sewage treatment; and in metallurgy. |
| Physical Properties | White hexagonal crystal; hygroscopic; density 3.16g/cm3; melts at 677°C; vaporizes at 1,023°C; vapor pressure 20 torr at 737°C and 200 torr at 897°C; highly soluble in water, ethanol and acetone; slightly soluble in benzene. The dihydrate and tetrahydrate are greenish monoclinic crystals; densities 2.39 and 1.39 g/cm3, respectively; decomposing at 120 and 105°C, respectively; both the hydrates soluble in water. |
| Preparation | Iron(II) chloride is prepared by passing chlorine or hydrogen chloride gas over iron at red heat or 700°C:
Fe + 2HCl → FeCl2 + H2
Fe + Cl2 → FeCl2
It also may be produced by the reduction of iron(III) chloride with hydrogen or other reducing agents at elevated temperatures:
2FeCl3 + H2 → 2FeCl2 + 2HCl
The tetrahydrate is obtained by dissolving the metal in hydrochloric acid followed by crystallization at room temperature.
Fe + 2HCl + 4H2O → FeCl2•4H2O + H2
The tetrahydrate gradually loses water when heated above 105°C forming dihydrate, monohydrate and the anhydrous salt. At 220°C it loses all its water of crystallization. |
| Chemical Properties | Greenish-white crystals. readily oxidized. Soluble in alcohol and water. |
| Chemical Properties | Ferrous chloride is a pale greenish salt-like crystal or power. |
| Physical properties | White hexagonal crystal; hygroscopic; density 3.16g/cm3; melts at 677°C;vaporizes at 1,023°C; vapor pressure 20 torr at 737°C and 200 torr at 897°C;highly soluble in water, ethanol and acetone; slightly soluble in benzene. Thedihydrate and tetrahydrate are greenish monoclinic crystals; densities 2.39and 1.39 g/cm3, respectively; decomposing at 120 and 105°C, respectively;both the hydrates soluble in water. |
| Uses | Ferrous chloride (FeCl2) is used in pharmaceutical preparations, for sewage treatment, and as a mordant (which fixes dyes so that they will not run) in textiles. |
| Uses | Used in the synthesis of a novel cis-Fe(BPE5)2Cl2 (BPE5=1,2-diphospholanoethane) complex, which has potential application in many types of reactions such as intra- or intermolecular activation.1 |
| Uses | A paramagnetic solid. |
| Definition | ChEBI: Iron dichloride is an iron coordination entity. |
| Preparation | Iron(II) chloride is prepared by passing chlorine or hydrogen chloride gasover iron at red heat or 700°C:
Fe + 2HCl → FeCl2 + H2
Fe + Cl2 → FeCl2
It also may be produced by the reduction of iron(III) chloride with hydrogenor other reducing agents at elevated temperatures:
2FeCl3 + H2 → 2FeCl2 + 2HCl
The tetrahydrate is obtained by dissolving the metal in hydrochloric acidfollowed by crystallization at room temperature.
Fe + 2HCl + 4H2O → FeCl2•4H2O + H2
The tetrahydrate gradually loses water when heated above 105°C formingdihydrate, monohydrate and the anhydrous salt. At 220°C it loses all its waterof crystallization. |
| General Description | Ferrous chloride is a greenish white crystalline solid. Ferrous chloride is soluble in water. Ferrous chloride is noncombustible. Ferrous chloride is used in sewage treatment, in dyeing of fabrics, and for many other uses. |
| Air & Water Reactions | Water soluble. |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
