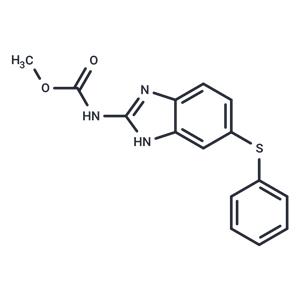
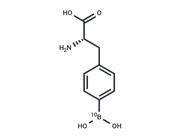
| Kinase Assay | Gelatin (0.1% (w/v) is added to standard LaemmLi acrylamide polymerization mixture. Tissue extract is mixed 1:2 with sample buffer [250 mM Tris-Cl pH 6.8, 10% (w/v) SDS, 20% (v/v) glycerol, 0.005% (w/v) bromphenol blue]. Serum is diluted 1:10 with electrophoresis buffer (2.5 mM Tris, 20 mM glycine, 0.005% SDS) and mixed 1:2 with sample buffer. Twenty μLs are loaded after 10-min incubation at room temperature without boiling. After electrophoresis at 90 V, the gels are soaked in 2.5% (w/v) Triton X-100, incubated 2 to 3 days at 37°C in gelatin digestion buffer [50 mM Tris-Cl, pH 8.0, 8 mM CaCl2, 10 mM ZnSO2, 0.02% (w/v) NaN3], stained in 0.05% Coomassie blue R-250 in acetic acid/methanol/water (1:4.5:4.5 by volume), destained in 10% acetic acid and 5% methanol, and scanned for lysis band intensity. The lysis band intensity is proportional to gelatinase activity and is quantified densitometrically by using One-Dimensional Scan software. The result, a number between 0.07 and 3.75, is normalized to the protein content by dividing the densitometry result with the relative optical density from the BCA protein assay kit result. The result is used for the analysis as the arbitrary unit. For the total MMP activity results of lysis bands of pro-MMP-9, active MMP-9, pro-MMP-2, and active MMP-2 are added. A protein size marker is used to determine the correct size. |

 United States
United States