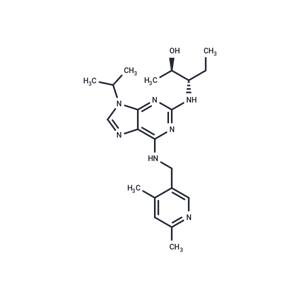
| Name | Fadraciclib |
| Description | Fadraciclib (CYC065) is an orally available, second-generation ATP-competitive inhibitor of CDK2/CDK9 kinases (IC50s: 5/26 nM). |
| Cell Research | Briefly, tumour cells are plated in six-well plates and treated with a titration of CYC065 concentrations (i.e., ranging from 100 to 500?nM). After 72?h, cells are harvested, washed and stained with propidium iodide (PI; 5?μg/mL) for flow cytometric counts. The percentage of viable cells is then normalized considering the vehicle-treated cells as 100% viable. Half-maximal inhibitory concentration values are determined using GraphPad Prism5 version 6. For drug combination studies, USC-ARK-1 and USC-ARK-2 cell lines are incubated with the combination of Taselisib and CYC065 at multiple paired concentrations including the IC50, the IC50/2 and the IC50*2 of each cell line to the corresponding drug (i.e., 10?nM of Taselisib and 198?nM of CYC065 for USC-ARK-1 and 50?nM of Taselisib and 62.5?nM of CYC065 for USC-ARK-2). Synergism is assessed by the combination index (CI). CI values <1 define a synergistic activity of the combination treatment. The CI values are calculated using the CompuSyn software [1]. |
| Animal Research | Briefly, 5-7-week-old SCID mice are injected into the subcutaneous region with USC cells. A minimum of five animals per group are used. Treatments are administrated by oral gavage starting 1 week after tumor implantation when the size of the tumor is 0.125-0.150?cm3. Uterine serous carcinoma-ARK-2-derived xenografts are divided into two groups: one group of animal receive the vehicle, whereas the experimental group receives CYC065 (22.5?mg/kg daily for 3 weeks). Uterine serous carcinoma-ARK-1-derived xenografts are instead divided into four groups: one group receive the vehicle (0.5% methylcellulose-0.2% Tween-80), one group receive CYC065 (22.5?mg/kg daily for 3 weeks), one group receive Taselisib (10 mg/kg daily, 5 days per week per 3 weeks) and the last group receive the combination of CYC065 and Taselisib. The size of the tumor at the initiation of treatment is 0.125-0.150?cm3. Mouse weight and tumor size is recorded two times a week for the entire experimental period. Tumor volume is calculated. |
| In vitro | CYC065 blocks cells in the G1 phase of the cell cycle and inhibits cell growth specifically in cyclin E1 (CCNE1)-overexpressing uterine serous carcinomas (USCs). USC cell lines expressing high CCNE1 mRNA and protein levels to be significantly more sensitive to treatment with CYC065 in vitro when compared with low CCNE1-expressing cell lines (IC50: mean±s.d.=124.1±57.8?nM in CCNE1-overexpressing USC cell lines vs 415±117.5?nM in CCNE1 low expressors, respectively; P=0.0003). Importantly, low concentrations of CYC065 (i.e., 100?nM) causes an arrest in the G1 phase of the cell cycle only in the CCNE1-overexpressing USC cell lines [1]. |
| In vivo | USC-ARK-2-derived xenografts are treated daily with CYC065 (22.5?mg/kg) for a 3-week period. Tumor size and mouse weight are recorded two times a week. The daily administration of CYC065 results in a significant reduction of tumor growth compared with the vehicle-treated mice (P=0.012 starting at day 9 of the treatment). No significant weight loss is reported during the entire treatment period [1]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 100 mg/mL (251.56 mM)
|
| Keywords | Inhibitor | inhibit | Fadraciclib | Cyclin dependent kinase | CYC-065 | CYC 065 | CDK |
| Inhibitors Related | Ribociclib | Ro-3306 | Rafoxanide | AT7519 | Palbociclib monohydrochloride | CASIN | Palbociclib | GW 441756 | Sodium Oxamate | Abemaciclib | Dinaciclib | Abemaciclib methanesulfonate |
| Related Compound Libraries | Bioactive Compound Library | ReFRAME Related Library | Kinase Inhibitor Library | Anti-Cancer Clinical Compound Library | Drug Repurposing Compound Library | Inhibitor Library | Orally Active Compound Library | Anti-Aging Compound Library | Bioactive Compounds Library Max | Cell Cycle Compound Library | Anti-Cancer Drug Library | Anti-Cancer Active Compound Library |

 United States
United States