WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
Product Information
Product Code: E068004
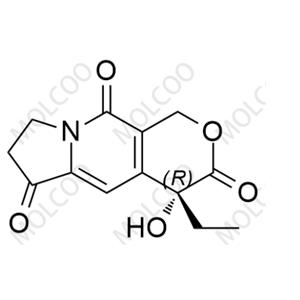
English Name: Exatecan Impurity 4
English Alias: (R)-4-ethyl-4-hydroxy-7,8-dihydro-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione
CAS Number: 110351-91-2
Molecular Formula: C13H13NO5
Molecular Weight: 263.25
Advantages:As a reference standard for Exatecan Impurity 4, it features a chemical structure confirmed by single-crystal X-ray diffraction and mass spectrometry, with ≥98.5% purity (HPLC), good stability at 2-8℃ in the dark, and high batch consistency. Suitable for impurity analysis of exatecan API and formulations, providing a reliable reference for quality control.
Applications:
Impurity Detection: Used to develop HPLC methods for detecting Impurity 4 in exatecan, determining LOD and LOQ, and controlling impurity content to meet ICH Q3A standards.
Process Optimization: Monitors the formation of Impurity 4 during exatecan synthesis, reducing its content by adjusting reaction conditions (e.g., temperature, solvent selection) to improve API purity.
Stability Studies: Evaluates the trend of Impurity 4 in accelerated stability tests (40℃/RH75%), providing data for storage conditions and shelf life.
Background Description:Exatecan is a topoisomerase I inhibitor for cancer treatment. Impurity 4 may be generated from cyclization reactions or oxidation byproducts during synthesis, and its presence may affect drug activity and safety. Therefore, strict control of Impurity 4 is a key part of exatecan's quality system.
Research Status:
Detection Technology: HPLC-UV with a C18 column (4.6×250mm, 5μm), mobile phase acetonitrile-0.1% phosphoric acid (45:55, v/v), detection at 254nm, with LOQ up to 0.05%.
Formation Mechanism: Impurity 4 may originate from cyclization side reactions of intermediates or subsequent oxidation steps, and optimizing cyclizing reagent dosage and reaction time can reduce its formation.
Safety Evaluation: Toxicological studies show the NOAEL of Impurity 4 in mice is 30mg/kg, and drug standards typically set its limit at ≤0.1%.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China


