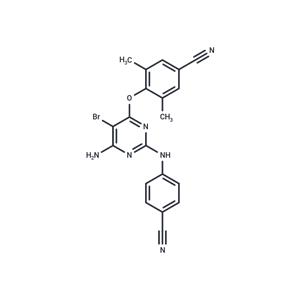
| Name | Etravirine |
| Description | Etravirine (R165335) is a diarylpyrimidine non-nucleoside reverse transcriptase inhibitor. Etravirine is designed to be active against HIV isolates with mutations that confer resistance to the two most commonly prescribed first-generation NNRTIs. It can bind the enzyme reverse transcriptase (RT) in multiple conformations, both for native and mutant RT, thereby blocking the enzymatic activity of RT. |
| In vitro | Etravirine (TMC125), is highly active against wild-type HIV-1 with EC50 of 1.4 nM to 4.8 nM and shows some activity against HIV-2 with EC50 of 3.5 μM. TMC125 also inhibits a series of HIV-1 group M subtypes and circulating recombinant forms and a group O virus. [1] [2] |
| In vivo | Etravirine (TMC125) demonstrates a high genetic barrier against resistance development and remains effective against HIV strains resistant to existing non-nucleoside reverse transcriptase inhibitors (NNRTIs), including those also resistant to protease inhibitors (PIs). Its tolerability profile, assessed in phase IIb trials with treatment-experienced patients, is comparable to the control group[3]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | Ethanol : < 1 mg/mL (insoluble or slightly soluble)
DMSO : 50 mg/mL (114.87 mM)
|
| Keywords | TMC 125 | Reverse Transcriptase | R 165335 | Inhibitor | Etravirine | HIV | Human immunodeficiency virus | TMC-125 | inhibit | R-165335 |
| Inhibitors Related | Stavudine | 5-Fluorouracil | Emtricitabine | Kaempferol | Dextran sulfate sodium salt (MW 4500-5500) | Lamivudine | Decanedioic acid |
| Related Compound Libraries | Bioactive Compound Library | Approved Drug Library | ReFRAME Related Library | EMA Approved Drug Library | Anti-Viral Compound Library | Drug Repurposing Compound Library | Inhibitor Library | FDA-Approved Drug Library | Bioactive Compounds Library Max | Anti-COVID-19 Compound Library |

 United States
United States