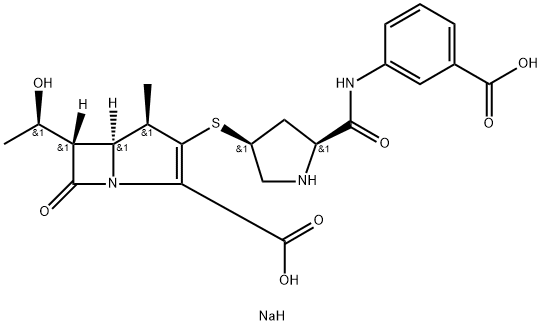
| CAS: | 153832-38-3 |
| MF: | C22H26N3NaO7S |
| MW: | 499.51 |
| EINECS: |
|
| Product Categories: | Aromatics;Chiral Reagents;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals;Sulfur & Selenium Compounds;API |
| Mol File: | 153832-38-3.mol |
 |
|
| Ertapenem Disodium Chemical Properties |
| Melting point | 230-234°C (dec.) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | Solid |
| color | White to Off-White |
| Stability: | Unstable at room temperature! |
| Ertapenem Disodium Usage And Synthesis |
| Description | Ertapenem sodium was introduced in the US as a once daily intravenous or intramuscular injection for the treatment of adult patients with moderate to severe bacterial infections. This new I-β-methyl carbapenem can be assembled from commercially available 4-nitrobenzyl protected β-methyl carbapenem enolphosphate and the appropriate thiol derivative. The last intermediate can be synthesized from a suitably N-protected 4- hydroxy proline derivative in a one pot operation involving bis activation of the carboxy and hydroxy groups, reaction with sodium sulfide yielding the corresponding thiolactone, aminolysis with 2-aminobenzoic acid and Kdeprotection. This bacterial cell wall synthesis inhibitor has broad spectrum antimicrobial activity including common Gram-positive and Gram-negative aerobic pathogens and restricted activity against nosocomial pathogens such as Pseudomonas aeruginosa, Acinetobacfer species, methicillin-resistant staphylococci and enterococci. Ertapenem is resistant to a broad and extended spectrum of β-lactamases (excluding metallo-beta-lactamase) and is also more resistant than imipenem to human renal dehydroxypeptidase-I-inactivation (DHP-I). In drug-resistant strains of P. aeruginosa, resistance to ertapenem and imipenem was common but almost all strains remain susceptible to at least one antipseudomal agent. In phase III studies, ertapenem showed efficacy in the treatment of obstetric and gyneacological infections, skin and soft tissues infections, community-acquired pneumonia, urinary tract infections and in intra abdominal infections. Ertapenem has improved pharmacokinetics over currently available carbapenems and cephalosporins with an extended serum half-life of 4h. The overall safety and tolerability profile of ertapenem was comparable to that seen with comparator antibacterials. |
| Chemical Properties | Pale Yellow Solid |
| Originator | Astra Zeneca (UK) |
| Uses | Group 1 carbapenem antibiotic. An antibacterial. |
| Brand name | lnvanz |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock


 Japan
Japan







 Company information
Company information Advantage
Advantage


