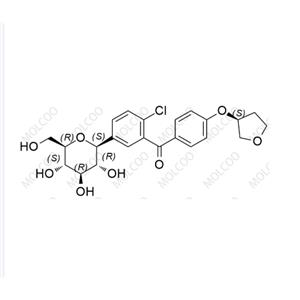
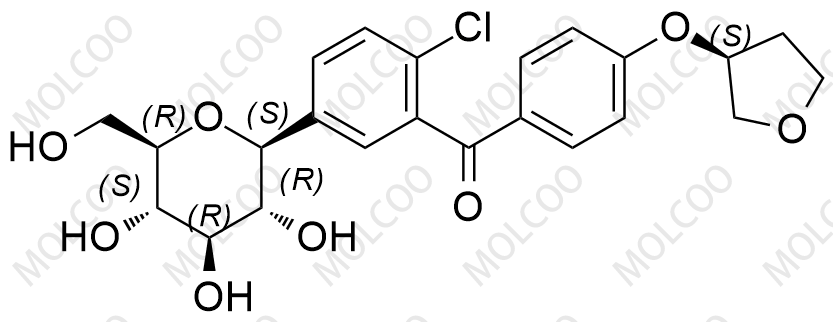
Empagliflozin Impurity YHT
Empagliflozin impurities refer to the unintended chemical components produced during the manufacturing process of empagliflozin, a hypoglycemic drug. These impurities may include various compounds with different structures.
The presence of empagliflozin impurities may have an important impact on the efficacy and safety of the drug. Therefore, in drug research and development and production processes, it is necessary to strictly control and analyze empagliflozin impurities to ensure the quality and safety of the drug.
We can provide a full range of impurity reference/standard products required for drug development. Most of the impurities are synthesized through the process, there are also many items of impurities can not be obtained by synthetic means, need to be obtained through raw materials, intermediates, crude products or side reactions contained in the trace target compounds, Hubei Moke has a professional impurity preparation and separation technology team, equipped with professional SFC preparation and separation equipment. It can carry out efficient and accurate separation of impurities for complex projects, and solve the problem of impurity preparation for customers.


 China
China