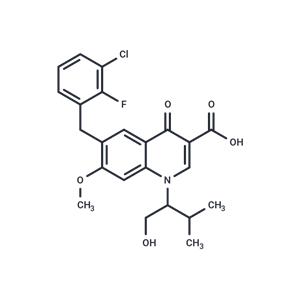
| Name | Elvitegravir |
| Description | Elvitegravir (JTK-303) is a Human Immunodeficiency Virus Integrase Strand Transfer Inhibitor. The mechanism of action of elvitegravir is as an HIV Integrase Inhibitor, and Cytochrome P450 2C9 Inducer. |
| Cell Research | Elvitegravir (EVG) is prepared in DMSO and stored, and then diluted with appropriate medium before use[1]. MT-2 cells (2×105 cells) are infected with HIV-1 IIIB and then cultured in the presence of 0.5 nM or 0.1 nM Elvitegravir. Cultures are incubated at 37°C until an extensive cytopathic effect (CPE) is observed, and the culture supernatant is then harvested for further passage in fresh MT-2 cells. The concentration of Elvitegravir is increased when a significant CPE is observed. At the indicated passages, proviral DNA is extracted from infected MT-2 cells and then subjected to PCR, followed by direct population-based sequencing. Susceptibility to Elvitegravir at the indicated passages is determined using the MAGI assay or p24 production[1]. |
| In vivo | Elvitegravir inhibits the replication of MLV infection with an IC50 of 5.8 nM and also suppresses the primate retrovirus SIV with an IC50 of 0.5 nM, demonstrating its activity against multiple retroviruses due to IN inhibitor properties. It inhibits integrase activity with an IC50 of 6 nM and affects PBMC and PA with IC50 values of 0.89 nM and 20 nM, respectively. Additionally, Elvitegravir blocks the synthesis of chain transfer products with an IC50 of 54 nM by inhibiting integration via IN-regulated chain transfer. EVG exhibits antiviral activity without serum, with IC50 values ranging from 0.3 to 0.9 nM, targeting peripheral blood mononuclear cells and effectively inhibiting HIV-1 and HIV-2. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 82 mg/mL (183.1 mM)
Ethanol : 33 mg/mL (73.7 mM)
H2O : < 1 mg/mL (insoluble or slightly soluble)
|
| Keywords | inhibit | HIV-1IIIB | HIV-2EHO | HIV | GS 9137 | JTK 303 | HIV Integrase | cDNA | D-06677 | Inhibitor | GS9137 | HIV-2ROD | JTK303 | Elvitegravir | D 06677 | Human immunodeficiency virus |
| Inhibitors Related | Stavudine | 5-Fluorouracil | Emtricitabine | Kaempferol | Dolutegravir intermediate-1 | Dextran sulfate sodium salt (MW 4500-5500) | Lamivudine | Chloroquine phosphate | Decanedioic acid | Tenofovir |
| Related Compound Libraries | Bioactive Compound Library | ReFRAME Related Library | Drug-induced Liver Injury (DILI) Compound Library | EMA Approved Drug Library | Drug Repurposing Compound Library | Anti-Viral Compound Library | Inhibitor Library | FDA-Approved Drug Library | Clinical Compound Library | Bioactive Compounds Library Max |

 United States
United States