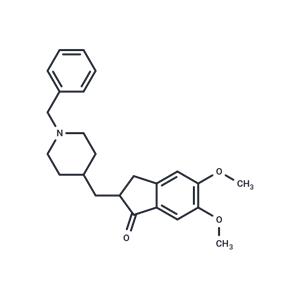
| Name | Donepezil |
| Description | Donepezil (Aricept) is a piperidine-based, potent, specific, and reversible inhibitor of acetylcholinesterase (AChE). It can be used for the treatment of mild to moderate dementia of the Alzheimer's type. |
| Cell Research | Cell lines: retinal ganglion cells (RGCs). Concentrations: 0.1-10 μM. Incubation Time: 3 days. RGC survival after exposure to each reagent (glutamate, donepezil, tacrine, galanthamine, and HA14-1) is measured by calcein-AM staining after 3 days in culture, Briefly, cells are incubated in 1 μM calcein-AM in PBS for 15 minutes at 37℃. After the medium is replaced with fresh PBS, cells are examined under a fluorescence microscope using a fluorescein filter. The total number of surviving RGCs defined as cells with a calcein-AM stained cell body and a process extending at least two cell diameters from the cell body is counted in each well. The number of surviving RGCs without any drug served as a control. |
| Animal Research | Animal Models: male C57BL/6 wild-type and CGRP(?/?) mice (10-12 weeks old, 21–24 g). Formulation: added to the food powder. Dosages: 1.5 mg/kg (i.g.) |
| In vitro | Donepezil has 500-1000-fold more selective for AChE over butyrylcholinesterase (BuChE). Short- and long-exposure of SH-SY5Y human neuroblastoma cells to donepezil induces a concentration-dependent inhibition of cell proliferation unrelated to muscarinic or nicotinic receptor blockade or apoptosis. Donepezil reduces the number of cells in the S-G2/M phases of the cell cycle, increases the G0/G1 population, and reduces the expression of two cyclins of the G1/S and G2/M transitions, cyclin E and cyclin B, in parallel with an increase in the expression of the cell cycle inhibitor p21. In addition, donepezil increases action potential-dependent dopamine release and modulates nicotinic receptors of substantia nigra dopaminergic neurons[1]. |
| In vivo | In plasma, urine, and bile, most donepezil metabolites are O-glucuronides. After oral ingestion, peak plasma concentrations are achieved in 3-5 hours and its absorption is not affected by food. Donepezil is slowly absorbed from the gastrointestinal tract and has a terminal elimination half-life of 50-70 hours in young volunteers (>100 hours in elderly subjects). After extensive metabolization in the liver, the parent compound is 93% bound to plasma proteins. Donepezil is metabolized in the liver via the cytochrome P450 system (CYP1A2-, CYP2D6-, CYP3A4-related enzymes). In animals, donepezil is found unchanged in the brain, and no metabolites are detected in the nervous tissue. Donepezil has linear pharmacokinetics over a dose range of 1-10 mg/day. 96% of circulating donepezil is protein bound [1]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 50 mg/mL (131.75 mM)
|
| Keywords | AChE | Donepezil | Cholinesterase (ChE) | Inhibitor | inhibit | hAChE | E 2020 | E-2020 | bAChE |
| Inhibitors Related | 1-Naphthyl acetate | DL-Menthol | Ethyl (triphenylphosphoranylidene) acetate | 4-Methylbenzylidene camphor | Isoeugenol acetate | Diethyltoluamide | Nizatidine | Methyl tridecanoate | Trimethylammonium chloride | Trimyristin |
| Related Compound Libraries | Bioactive Compound Library | Anti-Neurodegenerative Disease Compound Library | Neuronal Signaling Compound Library | Drug-induced Liver Injury (DILI) Compound Library | Drug Repurposing Compound Library | Inhibitor Library | NO PAINS Compound Library | FDA-Approved Drug Library | Orally Active Compound Library | Bioactive Compounds Library Max |

 United States
United States