Product Information
Product Number: D044002B
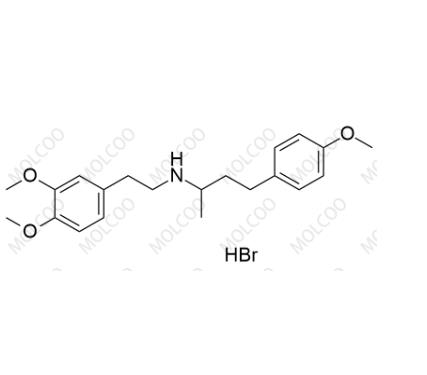
English Name: Dobutamine EP Impurity C(Hydrobromide)
English Alias: N-(3,4-dimethoxyphenethyl)-4-(4-methoxyphenyl)butan-2-amine hydrobromide
CAS Number: None
Molecular Formula: C₂₁H₂₉NO₃·HBr
Molecular Weight: 424.37(343.46 + 80.91)
Advantages
Well-defined structure and stable salt form, enabling analysis of by-product formation mechanisms during dobutamine synthesis, such as benzene ring substitution and amination reactions, to optimize processes and control impurity generation;
As a salt-form reference standard containing polymethoxybenzene rings and amino groups, it provides a stable quantitative standard for HPLC, LC-MS, and other detection methods, improving method accuracy;
The hydrobromide form enhances water solubility, facilitating simulation of impurity behavior in physiological environments during formulation stability studies.
Applications
Drug Development: Used as an impurity reference standard to identify and quantify Impurity C in dobutamine preparations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity and specificity of detection methods (e.g., HPLC or LC-MS), ensuring compliance with EP pharmacopoeia requirements;
Stability Studies: Investigating the degradation behavior of hydrobromide under acidic/alkaline conditions to evaluate its impact on dobutamine injection stability.
Background Description
Research Status
Detection Method Optimization: Establishing trace detection methods using UPLC-MS/MS technology, leveraging the ionization characteristics of hydrobromide to enhance mass spectrometry response sensitivity;
Synthesis Process Improvement: Reducing by-product generation by optimizing the catalyst (such as palladium carbon) and reaction temperature of methoxylation reactions;
Salt Form Stability: Studying the hygroscopicity and degradation pathways of hydrobromide under high temperature and humidity conditions to provide data for storage conditions;
Toxicological Evaluation: Evaluating the potential impact of this impurity on cardiac muscle cells through in vitro cytotoxicity experiments to assist in setting safe limits.





 China
China