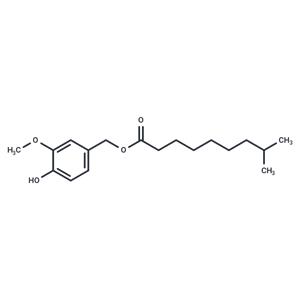
| In vitro | Dihydrocapsiate treatment at concentrations of 10, 25, and 50 μM for 48 hours does not impact the viability of human preadipocytes, indicating its non-toxic nature at these levels [1]. At lower concentrations of 10 and 20 μM, but over a longer period of 8 days, dihydrocapsiate significantly reduces the expression of various adipogenic (like SREBP1, FABP4, PLIN1, ADIPOQ, and LEPTIN) and inflammatory markers (MCP1 and TNFα) in mature adipocytes. Conversely, it increases the expression of PGC1α, crucial for mitochondrial biogenesis, and TBX1, a marker of "brite" cells, highlighting its potential role in adipocyte metabolism and inflammation modulation [1]. Interestingly, in RAW 264.7 cells, dihydrocapsiate concentrations ranging from 25 to 200 μM effectively inhibit the release of nitric oxide (NO) and the generation of intracellular reactive oxygen species (ROS), providing evidence of its anti-inflammatory and antioxidative properties [1]. |
| In vivo | Dihydrocapsiate administered orally at doses of 2 and 10 mg/kg effectively improves morphometric parameters and insulin levels in HFD-fed mice [1]. It counteracts the enlargement of adipocytes and upregulates energy expenditure-related genes in white adipose tissue (WAT), mitigates hepatic steatosis, and inhibits fat accumulation prompted by a high-fat diet (HFD). Furthermore, it elevates the expression of mitochondrial biogenesis-related genes in brown adipose tissue (BAT), ameliorates intestinal morphology, and modulates the availability of short-chain fatty acids (SCFAs), showcasing its broad pharmacological potential in metabolic regulation. |

 United States
United States