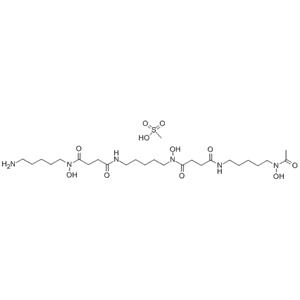
| Description | Deferoxamine mesylate is an iron chelator that binds free iron in a stable complex, preventing it from engaging in chemical reactions. |
|---|
| Related Catalog | Signaling Pathways >> Neuronal Signaling >> Amyloid-β Signaling Pathways >> Autophagy >> Autophagy Signaling Pathways >> Autophagy >> Mitophagy Research Areas >> Others |
|---|
| In Vitro | Deferoxamine treatment significantly increases HIF-1α binding under all culture conditions, including hypoxic and high-glucose. The mechanism of deferoxamine is through improving HIF-1α biological function through scavenging oxygen free radicals[1]. Deferoxamine (5 μM) has significant effect on the tumor-associated stromal cells cellular multiplication, and cells die at day 7 after exposure to 50 μM and 100 μM deferoxamine. Deferoxamine (5 μM-100 μM) inhibits the proliferation of BMMSCs, and induces apoptosis of MSCs in a dose-dependent manner. Deferoxamine influences the expression of adhesion proteins on MSCs[3]. Deferoxamine (30, 60, 120 μM) shows lower expression of HIF-1α in a concentration dependent way in AdMSCs[4]. |
|---|
| In Vivo | Deferoxamine (100 mg/kg, i.p.) lowers the mortality rate of subarachnoid hemorrhage (SAH) rat. Deferoxamine (100 mg/kg, i.p.) attenuates Evan’s blue extravasation in cortex, ameliorates the tight junction detachment and preserves the integrity of the base membrane examined in electron microscope at day 3 after SAH. Deferoxamine attenuates degradation of BBB proteins after SAH and significantly reduces ferritin expression at day 3 in the cortex, and improves neurologic behavior and cognitive deficits after experimental[1]. Ten µL of 1 mM deferoxamine-treated wounds display significantly accelerated healing from day 7 onward and heal significantly faster than control-treated wounds in diabetic mice. Deferoxamine-treated wounds and dimethyloxalylglycine-treated wounds heal significantly faster than control-treated wounds in aged mice[2]. In deferoxamine (10 mg/mL)-treated TG mice, there is a decrease in both soluble and insoluble Aβ40 and Aβ42. Both pGSK3β and β-catenin are significantly increased by approximately 50% in the deferoxamine-treated mice[5]. |
|---|
| Cell Assay | Harvested murine tumor and bone marrow-derived MSCs are exposed to varying doses of deferoxamine. Cell viability is assessed by trypan blue exclusion assay. The viable cells are more than 98% before enrolled for experiments. A total of 1.5×105 TAMSCs/well or 3×105 BMMSCs/well are seeded in 6-well plates. Then MSCs are exposed to 5, 10, 25, 50, and 100 μM deferoxamine on the following day. After 7 days, the number of TAMSCs is counted. To assess the cytotoxicity of deferoxamine to primary bone marrow MSCs, 2×106 bone marrow cells/well are seeded in 24-well plates. After 9 days, the number of survival cells is counted. To assess the cell cycle, TAMSCs are stained with propidium iodide, and cell cycle distribution is analyzed by flow cytometry. |
|---|
| Animal Admin | Mice are divided into three treatment groups of 17 each: (1) TG mice given IN Deferoxamine (TG-DFO), (2) TG mice given IN phosphate buffered saline (TG-PBS), and (3) WT mice given IN PBS (WT-PBS). At 30 weeks of age, mice are acclimated to handling and then treated intranasally every monday, wednesday, and friday, starting at 36 weeks of age. Mice are dosed for 18 weeks, until behavior tests at 54 weeks. Dosing continues during the 4 weeks of behavior to measure both chronic and acute effects. After behavior mice are dosed a final time, and 24 h later euthanized and tissues collected for biochemical analyses. These include soluble and insoluble amyloid as measured by ELISA and IHC, quantification of proteins with Western blot and oxidative markers. |
|---|
| References | [1]. Li Y, et al. Effects of deferoxamine on blood-brain barrier disruption after subarachnoid hemorrhage. PLoS One. 2017 Mar 1;12(3):e0172784 [2]. Duscher D, et al. Comparison of the Hydroxylase Inhibitor Dimethyloxalylglycine and the Iron Chelator Deferoxamine in Diabetic and Aged Wound Healing. Plast Reconstr Surg. 2017 Mar;139(3):695e-706e [3]. Wang G, et al. In vitro assessment of deferoxamine on mesenchymal stromal cells from tumor and bone marrow. Environ Toxicol Pharmacol. 2017 Jan;49:58-64 [4]. Wahl EA, et al. VEGF released by deferoxamine preconditioned mesenchymal stem cells seeded on collagen-GAG substrates enhances neovascularization. Sci Rep. 2016 Nov 10;6:36879 [5]. Fine JM, et al. Intranasal deferoxamine engages multiple pathways to decrease memory loss in the APP/PS1 model of amyloid accumulation. Neurosci Lett. 2015 Jan 1;584:362-7 |
|---|

 China
China