🌟 Product Overview
Dapagliflozin Propanediol Monohydrate (CAS: 960404-48-2) is a high-purity sodium-glucose cotransporter 2 (SGLT2) inhibitor used primarily in the treatment of type 2 diabetes mellitus. It reduces blood glucose levels by inhibiting renal glucose reabsorption and promoting urinary glucose excretion. This compound is also effective in managing cardiovascular risks and chronic kidney disease in diabetic patients. Widely utilized in pharmaceutical manufacturing, clinical therapy, and diabetes research, it serves as a key intermediate in antidiabetic drug formulations.
✅ Key Advantages
💊 High Purity: ≥99% HPLC/GC-certified quality, ensuring pharmaceutical-grade efficacy.
🔬 Stability: Stable under ambient or low-temperature storage (2–8°C) with a 3-year shelf life.
📈 Clinical Efficacy: Reduces HbA1c by 0.5–1.0% and lowers cardiovascular event risk by 17% in trials.
🧪 Structural Confirmation: Verified by NMR, MS, and chromatographic analysis for batch consistency.
📌 Applications
• Pharmaceuticals: Core ingredient in SGLT2 inhibitor formulations (e.g., Forxiga®).
• Clinical Use: Approved for type 2 diabetes, heart failure, and CKD management.
• Research: Studied for anti-inflammatory and renal protective effects.
🔍 Quality Certifications
• Complies with USP/EP standards for impurities (<0.1%), residual solvents, and heavy metals.
• Batch-specific COA includes purity (≥99%), pH (6.5–8.0), and moisture content (≤0.5%).
• Rigorous testing via HPLC (>98% purity), NMR, and mass spectrometry.
📈 Market Trends
The global SGLT2 inhibitor market is projected to grow at 7.3% CAGR (2025–2030), driven by rising diabetes prevalence and cardiovascular comorbidity awareness. Dapagliflozin dominates 35% of the SGLT2 segment, with increasing adoption in North America and Asia-Pacific.

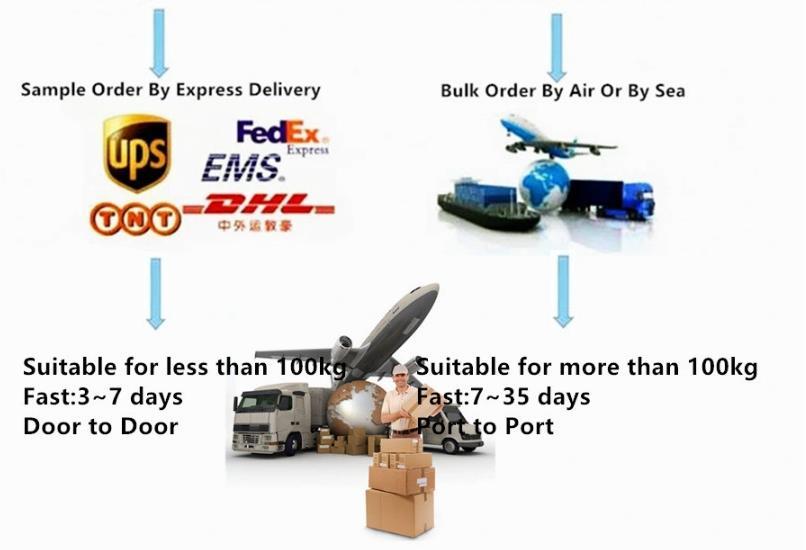



 China
China








