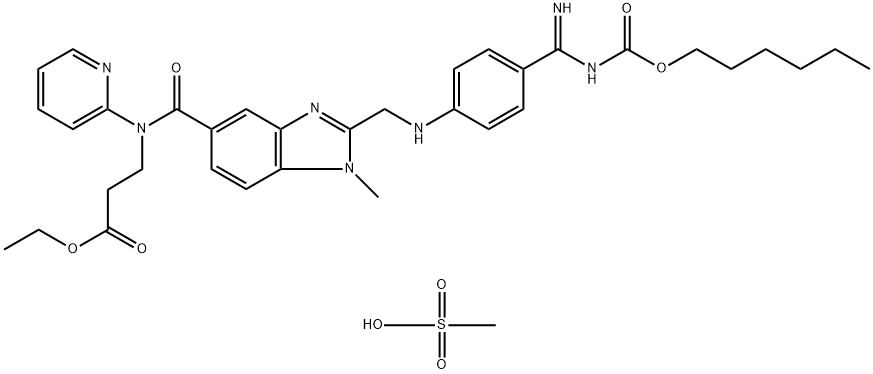
| CAS: | 872728-81-9 |
| MF: | C35H45N7O8S |
| MW: | 723.85 |
| EINECS: | 828-727-6 |
| Product Categories: | Aromatics;Bases & Related Reagents;Heterocycles;Intermediates & Fine Chemicals;Nucleotides;Pharmaceuticals;Dabigatran etexilate mesylate;API;872728-81-9 |
| Mol File: | 872728-81-9.mol |
 |
|
| Dabigatran Etexilate Mesylate Chemical Properties |
| Melting point | >125°C |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly) |
| form | Solid |
| color | White to Pale Yellow |
| Dabigatran Etexilate Mesylate Usage And Synthesis |
| Description | Dabigatran etexilate mesylate is a clinical direct thrombin inhibitor and a new type of oral anticoagulant. It can inhibit the formation of thrombus by reversibly and potently competing with the fibrin-specific binding site of thrombin to block fibrin generation. |
| Chemical Properties | Off-White to Pale Yellow Solid |
| Uses | Nonpeptide, direct thrombin inhibitor. Antithrombotic. |
| Definition | ChEBI: A methanesulfonate salt obtained by reaction of dabigatran etexilate with one equivalent of dabigatran etexilate. A prodrug for dabigatran, a thrombin inhibitor and anticoagulant which is used for the prevention of stroke and systemic embolism. |
| Biological Activity | Dabigatran etexilate mesylate (BIBR 1048MS) is an orally active prodrug of Dabigatran. Dabigatran etexilate mesylate has anticoagulant properties and can prevent venous thromboembolism and stroke due to atrial fibrillation. |
| Mechanism of action | Dabigatran etexilate mesylate is a prodrug of dabigatran that is metabolized in the body and converted to the active dabigatran. Compared with warfarin, dabigatran etexilate mesylate does not require frequent monitoring of coagulation function and dose adjustment during treatment, and there are fewer interactions between drugs and is not affected by eating, thus improving patients' medication compliance. |
| in vivo | Dabigatran etexilate mesylate (BIBR 1048MS; oral; 10, 20 and 50 mg/kg for rats and 1, 2.5 and 5 mg/kg for monkeys) has dose- and time-dependent anticoagulant effects and has maximum effects between 30 and 120 min after administration, respectively.Dabigatran etexilate mesylate maximally and significantly prolongs partial thromboplastin time (aPTT) to 25.2, 38.4 and 78.3 s in 30 min after 10, 20 and 50 mg/kg oral doses, respectively.Dabigatran etexilate mesylate maximally prolongs the aPTT to 34.3 s, 44.0 s, and 63.0 s, respectively, 2h after 1, 2.5 or 5 mg/kg doses in the monkey. |
Product picture
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock


 Japan
Japan








 Company information
Company information Advantage
Advantage


