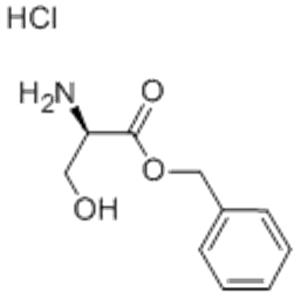
Peptide Synthesis: D-Ser-Obzl·HCl serves as a building block for the synthesis of peptides. The benzyl ester (Obzl) protecting group stabilizes the serine residue during the synthetic process, enabling efficient coupling reactions. This reagent can be used to introduce D-serine into peptide chains, either as a starting amino acid or as an intermediate during chain elongation. Stereoselective Synthesis: The stereoselectivity of D-Ser-Obzl·HCl is crucial in peptide synthesis. The D-configuration of the serine residue ensures that the desired chirality is maintained throughout the synthesis process. This stereoselectivity is essential for the production of peptides with specific biological activities and functions.
Biochemical Studies: Biochemists often utilize D-Ser-Obzl·HCl in their research to investigate the role of D-serine and its analogs in biological systems. D-serine is an important neuromodulator in the central nervous system, playing a crucial role in synaptic transmission and neural plasticity. By incorporating D-Ser-Obzl·HCl into peptides or proteins, researchers can gain insights into the functional roles of D-serine in cells and organisms.
Drug Discovery and Development: The unique biological activity of D-serine and its analogs has attracted interest in their potential as therapeutic agents. D-Ser-Obzl·HCl, as a synthetic intermediate, could be explored in drug discovery efforts. Its ability to modulate specific biological processes or interact with target molecules could lead to the identification of novel therapeutic strategies.
Structural Studies: The benzyl ester protecting group in D-Ser-Obzl·HCl can be removed under appropriate conditions, revealing the free hydroxyl group of the serine residue. This allows for further derivatization or functionalization, enabling structural studies and the exploration of novel biological activities. | 
 China
China