1/5
CYPROHEPTADINE HYDROCHLORIDE NEW
- Min. Order1KG
- Purity99%
- Cas No969-33-5
- Supply Ability50000KG/month
- Update time2025-03-21

| Product Name | CYPROHEPTADINE HYDROCHLORIDE |
| CAS No | 969-33-5 |
| EC-No | 213-535-1 |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 50000KG/month |
| Release date | 2025/03/21 |
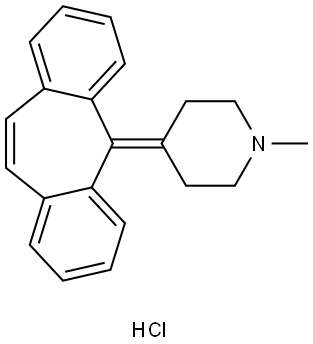
| CAS: | 969-33-5 |
| MF: | C21H22ClN |
| MW: | 323.86 |
| EINECS: | 213-535-1 |
| Product Categories: | Serotonin receptor;Active Pharmaceutical Ingredients |
| Mol File: | 969-33-5.mol |
 | |
| CYPROHEPTADINE HYDROCHLORIDE Chemical Properties |
| Melting point | 254-256.5 °C(Solv: ethanol (64-17-5); ethyl ether (60-29-7)) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | ethanol: soluble |
| form | solid |
| color | Crystals from EtOH/Et2O |
| Water Solubility | Soluble in water (15mM) |
| Stability: | Hygroscopic |
| CYPROHEPTADINE HYDROCHLORIDE Usage And Synthesis |
| Originator | Periactin,Merck Sharp and Dohme,US,1961 |
| Uses | Antipruritic;5-HT antagonist |
| Uses | Cyproheptadine hydrochloride is an antihistamine in which antiserotonin activity has been demonstrated both in vivo and in vitro. As yet, however, there is no evidence that this action contributes to clinical therapeutic effects. Anticholinergic and sedative effects are observed. Cyproheptadine may be more effective than other H1 blockers in the management of cold urticaria. |
| Definition | ChEBI: The hydrochloride salt of cyproheptadine. Note that the drug named cyproheptadine hydrochloride generally refers to cyproheptadine hydrochloride sesquihydrate. |
| Manufacturing Process | (A) Preparation of 1-Methyl-4-Piperidyl-Magnesium Chloride: Magnesium turnings (5.45 g, 0.22 g-atom) were placed in a 500 ml 3-necked flask provided with a condenser, Hershberg stirrer and dropping funnel and protected with a drying tube. An atmosphere of dry nitrogen was maintained in the apparatus throughout the reaction. The magnesium was covered with 20 ml of dry tetrahydrofuran. A crystal of iodine and 1.2 g of ethyl bromide were added and after the reaction had subsided (formation of ethylmagnesium bromide) a solution of 29.4 g (0.22 mol) of 4-chloro-1-methyl-piperidine in dry tetrahydrofuran (total volume, 103 ml) was added dropwise at such a rate that gentle reflux was maintained. The solution of 4-chloro-1-methylpiperidine in tetrahydrofuran was dried over calcium hydride at ice-bath temperature prior to use. When the addition of the halide was complete the reaction mixture was refluxed with stirring for one hour. In some subsequent experiments this period of refluxing was omitted with no deleterious result. The solution of 4-chloro-1-methylpiperidine in tetrahydrofuran was dried over calcium hydride at ice-bath temperature prior to use. When the addition of the halide was complete the reaction mixture was refluxed with stirring for one hour. In some subsequent experiments this period of refluxing was omitted with no deleterious result. The solvent was evaporated from the combined benzene extracts to give 33.4 g of a clear light brown resin. Crystallization from an alcohol-water mixture gave 19.5 g of 1-methyl-4-(5-hydroxy-5-dibenzo[a,e]cycloheptatrienyl)- piperidine, MP 156° to 157°C. Two recrystallizations from alcohol-water mixtures followed by two recrystallizations from benzene-hexane mixtures gave analytically pure product, MP 166.7° to 167.7°C. (C) Preparation of 1-Methyl-4-(5-Dibenzo[a,e]Cycloheptatrienylidene)- Piperidine Hydrochloride: 1-Methyl-4-(5-hydroxy-5-dibenzo[a,e] cycloheptatrienyl)-piperidine (3.05 g, 0.01 mol) was dissolved in glacial acetic acid, 15 ml. The solution was saturated with dry hydrogen chloride with external cooling. A white solid separated. Acetic anhydride (3.07 g, 0.03 mol) was added and the mixture heated on the steam bath for one hour. The solid dissolved in the first 5 minutes of the heating period. The reaction mixture was poured into 25 ml of water and the mixture made strongly basic with 10N sodium hydroxide solution. The mixture was extracted 3 times with 50 ml portions of benzene, the combined extracts washed with water and concentrated to a volume of approximately 50 ml. The solution was saturated with dry hydrogen chloride and the white crystalline product collected and dried. The yield of product, MP 251.6° to 252.6°C (dec.) was 2.5 g. Recrystallization from a mixture of absolute alcohol and absolute ether gave a product, MP 252.6° to 253.6°C. A sample was analyzed after drying for 7 hours at 110°C over phosphorus pentoxide in vacuo. (D) Preparation of 1-Methyl-4-(5-Dibenzo[a,e]Cycloheptatrienylidene)- Piperidine: The hydrochloride salt, 4.3 g, was suspended in 100 ml of warm water and the mixture made strongly alkaline by the addition of 15 ml of 5% sodium hydroxide. The mixture was extracted with four 50 ml portions of benzene and the extracts dried over sodium sulfate. Evaporation of the benzene on the steam-bath at reduced pressure left 3.7 g (97%) of the base,MP 110.3° to 111.3°C. Recrystallization from a mixture of alcohol and water gave product, MP 112.3° to 113.3°C. |
| Brand name | Periactin (Merck). |
| Therapeutic Function | Antipruritic, Antihistaminic, Appetite stimulant |
| General Description | Cyproheptadinehydrochloride, 4-(5H-dibenzo-[a,d]-cyclohepten-5-ylidine)-1-methylpiperidine hydrochloride sesquihydrate(Periactin), is slightly soluble in water and sparingly solublein alcohol. Cyproheptadine possesses both antihistamine and antiserotoninactivity and is used as an antipruritic agent. It isindicated for the treatment of hypersensitivity reactions,perennial, and seasonal allergic rhinitis; vasomotor rhinitis;allergic conjunctivitis, uncomplicated allergic skin manifestationsof urticaria and angioedema; amelioration of allergicreactions to blood or plasma; and cold urticaria. It is alsoused off-label for nightmares associated with posttraumaticstress disorder (PTSD), prevention of migraine, suppressionof vascular headaches, and appetite stimulation. Sedation isthe most prominent side effect, and this is usually brief, disappearingafter 3 or 4 days of treatment. |
| Biological Activity | Non-selective 5HT 2 antagonist, migraine prophylactic. |
| Safety Profile | Poison by ingestion and intraperitoneal routes. Human systemic effects by ingestion: jaundice, liver function tests impaired, gastrointestinal effects. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. Whenheated |
| Drug interactions | Potentially hazardous interactions with other drugs Analgesics: sedative properties increased with opioid analgesics. |
| Metabolism | Undergoes almost complete metabolism in the liver. The main metabolite found in humans is a quaternary ammonium glucuronide conjugate of cyproheptadine. 40% is excreted in the urine mainly as metabolites and 2-20% via the faeces. |
| storage | Room temperature |
| references | [1] levine b, green-johnson d, hogan s, smialek je. a cyproheptadine fatality. j anal toxicol. 1998 jan-feb;22(1):72-4. [2] lin oa, karim za, vemana hp, espinosa ev, khasawneh ft. the antidepressant 5-ht2a receptor antagonists pizotifen and cyproheptadine inhibit serotonin-enhanced platelet function. plos one. 2014 jan 23;9(1):e87026. |
Product picture

Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
-







