1/5
Cyclohexanone NEW
- Min. Order1KG
- Purity99%
- Cas No108-94-1
- Supply Ability50000KG/month
- Update time2025-08-08



| Product Name | Cyclohexanone |
| CAS No | 108-94-1 |
| EC-No | 203-631-1 |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 50000KG/month |
| Release date | 2025/08/08 |
| CAS: | 108-94-1 |
| MF: | C6H10O |
| MW: | 98.14 |
| EINECS: | 203-631-1 |
| Product Categories: | Alphabetical Listings;C-D;Alpha Sort;C;CAlphabetic;Flavors and Fragrances;CO - CZ;Volatiles/ Semivolatiles;Analytical Reagents for General Use;C-D, Puriss p.a.;Puriss p.a.;ACS GradeCarbonyl Compounds;C3 to C6;Essential Chemicals;Intermediates of Dyes and Pigments;Ketones;Routine Reagents;Reagent Plus;Analytical/Chromatography;Auxiliaries for ISE;Ion Sensor Materials;Halogenated Heterocycles ,Indolines ,Indoles ,Indazoles;pharmaceutical intermediate;1;108-94-1 |
| Mol File: | 108-94-1.mol |
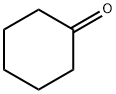 | |
| Cyclohexanone Chemical Properties |
| Melting point | -47 °C (lit.) |
| Boiling point | 155 °C (lit.) |
| density | 0.947 g/mL at 25 °C (lit.) |
| vapor density | 3.4 (vs air) |
| vapor pressure | 3.4 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 3909 | CYCLOHEXANONE |
| Fp | 116 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 90g/l |
| pka | 17(at 25℃) |
| form | Liquid |
| color | APHA: ≤10 |
| PH | 7 (70g/l, H2O, 20℃) |
| Relative polarity | 0.281 |
| Odor | Like peppermint and acetone. |
| Odor Type | minty |
| explosive limit | 1.1%, 100°F |
| Water Solubility | 150 g/L (10 ºC) |
| Merck | 14,2726 |
| JECFA Number | 1100 |
| BRN | 385735 |
| Henry's Law Constant | 1.2 x 10-5 atm?m3/mol at 25 °C (Hawthorne et al., 1985) 6.92 x 10-5 atm?m3/mol at 60.00 °C, 10.7 at 70.00 °C, 16.4 at 80.00 °C (headspace-GC, Hovorka et al., 2002) |
| Exposure limits | TLV-TWA 100 mg/m3 (25 ppm) (ACGIH); IDLH 5000 ppm (NIOSH). |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| LogP | 0.86 at 25℃ |
| CAS DataBase Reference | 108-94-1(CAS DataBase Reference) |
| IARC | 3 (Vol. 47, 71) 1999 |
| NIST Chemistry Reference | Cyclohexanone(108-94-1) |
| EPA Substance Registry System | Cyclohexanone (108-94-1) |
| Cyclohexanone Usage And Synthesis |
| Chemical properties | Cyclohexanone is a colorless, clear liquid with soil smell; its impure product appears as light yellow color. It is miscible with several other solvents. easily soluble in ethanol and ether. The lower exposure limit is 1.1% and the upper exposure limit is 9.4%. Cyclohexanone may be incompatible with oxidizers and nitric acid. Cyclohexanone is a primarily used in industry, up to 96%, as a chemical intermediate in the production of nylons 6 and 66. Oxidation or conversion of cyclohexanone yields adipic acid and caprolactam, two of the immediate precursors to the respective nylons. Cyclohexanone can also be used as a solvent in a variety of products, including paints, lacquers, and resins. It has not been found to occur in natural processes. |
| Application | Cyclohexanone is mostly captively consumed, either isolated or as a mixture, in the production of nylon intermediates (adipic acid and Caprolactam). Around 4% is consumed in markets other than nylon, such as solvents for paints, dyes and pesticides. Cyclohexanone is also used in the manufacture of pharmaceuticals, films, soaps and coatings. Fibrant is one of the largest producers of Cyclohexanone, a raw material used in the production of Caprolactam. |
| Uses | Cyclohexanone is an important chemical raw material, being the major intermediates of making nylon, caprolactam and adipic acid. It is also an important industrial solvent, for example, for paints, especially for those containing nitrocellulose, vinyl chloride polymers and their copolymers or methacrylate polymer paints. Used as an excellent solvent for pesticides such as organophosphate insecticide. It is used as a solvent for dyes, viscous solvents for piston aviation lubricants, solvents for greases, waxes and rubbers. Also used as leveling agent for dyeing and fading silk; degreasing agents for polishing metal; wood coloring paint; also used for cyclohexanone stripping, decontamination and spot removal. Cyclohexanone and cyanoacetic acid can have condensation reaction to generate cyclohexylidene acetic acid, and then followed by elimination and decarboxylation to get cyclohexene acetonitrile, and finally giving cyclohexene ethylamine by hydrogenation [3399-73-3]. Cyclohexene ethylamine is a intermediate for some drugs. |
| Production method | In the 1940s, the industrial production of cyclohexanone mainly applied hydrogenation of phenol to generate cyclohexanol, followed by dehydrogenation to give cyclohexanone. In the 1960s, with the development of petrochemical industry, the cyclohexane oxidation production method gradually dominated. In 1967, the one step method of phenol hydrogenation, developed by the Netherlands National Mining Company (DSM) was industrialized. This method has short production process, good product quality and high yield, but the raw materials of phenol and catalyst are expensive, so the majority of the industry still adopts the cyclohexane oxidation method. 1. Phenol method takes nickel as a catalyst; first apply hydrogenation of phenol to give cyclohexanol, followed by dehydrogenation to give cyclohexanone using zinc as the catalyst for zinc. 2. Cyclohexane oxidation method uses cyclohexane as the raw material; first apply non-catalyst condition; use oxygen-rich air for oxidation to give cyclohexyl hydroperoxide, followed by decomposition into the mixture of cyclohexanol, cyclohexanone, alcohol and ketone in the presence of tert-butyl chromate catalyst; further apply a series of distillation refinement to get qualified products. Raw material consumption quota: cyclohexane (99.6%) 1040kg / t. 3. Benzene hydrogenation oxidation method; benzene subjects to hydrogenation (with hydrogen) at 120-180 ℃ in the presence of nickel catalyst to generate cyclohexane; cyclohexane has oxidation reaction with air at 150-160 ℃, 0.908MPa to obtain the mixture of cyclohexanol and cyclohexanone; separate them to obtain the cyclohexanone product. Cyclohexanol is dehydrogenated at 350-400 ° C in the presence of a zinc-calcium catalyst to produce cyclohexanone. Raw material consumption quotas: benzene (99.5%) 1144kg / t, hydrogen (97.0%) 1108kg / t, caustic soda (42.0%) 230kg / t. |
| Description | Cyclohexanone, a colorless liquid is a cyclic ketone. It is an important building block for the synthesis of a variety of organic compounds. Majority of the cyclohexanone synthesized is utilized as an intermediate in the synthesis of nylon. Used as a polyvinyl chloride (PVC) solvent, cyclohexanone caused contact dermatitis in a woman manufacturing PVC fluidotherapy bags. Cyclohexanone probably does not cross react with cyclohexanone resin. A cyclohexanone-derived resin used in paints and varnishes caused contact dermatitis in painters.  |
| Chemical Properties | Cyclohexanone is a water-white to slightly yellow liquid with a peppermint-like or acetone-like odor. The Odor Threshold is 0.12 0.24 ppm in air. |
| Physical properties | Clear, colorless to pale yellow, oily liquid with a peppermint-like odor. Experimentally determined detection and recognition odor threshold concentrations were identical: 480 μg/m3 (120 ppmv) (Hellman and Small, 1974). |
| Occurrence | Reported present in Cistus labdaniferus. |
| Uses | Industrial solvent for cellulose acetate resins, vinyl resins, rubber, and waxes; solventsealer for polyvinyl chloride; in printing industry; coating solvent in audio and videotape production |
| Uses | Cyclohexanone is used as an industrial solvent and paint remover. It acts as a precursor to nylon 6,6 and nylon 6 and cyclohexanone oxime, which gives caprolactam on rearrangement. Further, it is used as a chemical reaction medium, adhesives, sealants and agricultural products. |
| Uses | Cyclohexanone is used in the productionof adipic acid for making nylon; in thepreparation of cyclohexanone resins; and asa solvent for nitrocellulose, cellulose acetate,resins, fats, waxes, shellac, rubber, and DDT.. |
| Definition | ChEBI: A cyclic ketone that consists of cyclohexane bearing a single oxo substituent. |
| Synthesis Reference(s) | Canadian Journal of Chemistry, 62, p. 1031, 1984 DOI: 10.1139/v84-171 Tetrahedron Letters, 25, p. 3309, 1984 DOI: 10.1016/S0040-4039(01)81371-X |
| General Description | A colorless to pale yellow liquid with a pleasant odor. Less dense than water . Flash point 111°F. Vapors heavier than air. Used to make nylon, as a chemical reaction medium, and as a solvent. |
| Air & Water Reactions | Flammable. Soluble in water. |
| Reactivity Profile | Cyclohexanone forms an explosive peroxide with H2O2, and reacts vigorously with oxidizing materials (nitric acid). |
| Health Hazard | Inhalation of vapors from hot material can cause narcosis. The liquid may cause dermatitis. |
| Health Hazard | The toxicity of cyclohexanone in test specieswas found to be low to moderate. Exposureto its vapors can produce irritation in the eyesand throat. Splashing into the eyes can damagethe cornea. Throat irritation in humansmay occur from 3–5 minute exposure to a50-ppm concentration in air. The symptomsof chronic toxicity in animals from its inhalationwere liver and kidney damage, as wellas weight loss. However, its acute toxicitywas low below 3000 ppm. The symptomsin guinea pigs were lacrimation, salivation,lowering of heart rate, and narcosis. Exposureto 4000 ppm for 4–6 hours was lethalto rats and guinea pigs. The oral toxicity of this compound waslow. Ingestion may cause narcosis and depressionof the central nervous system. It canbe absorbed through the skin. LD50 value, dermal (rabbits): 1000 mg/kg LD50 value, intraperitoneal (rats): 1130 mg/kg. |
| Fire Hazard | HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water. |
| Flammability and Explosibility | Flammable |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
-






 China
China

