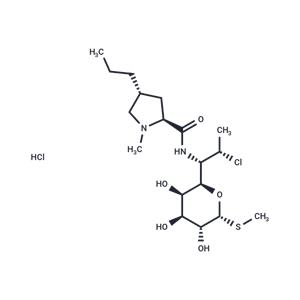
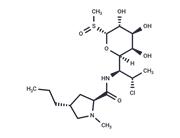
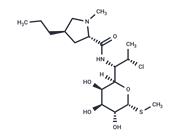
| Name | Clindamycin hydrochloride |
| Description | Clindamycin hydrochloride (Clinimycin HCl) inhibits protein synthesis by acting on the 50S ribosomal. It is the hydrochloride salt form of clindamycin, a semi-synthetic, chlorinated broad-spectrum antibiotic produced by chemical modification of lincomycin. Clindamycin hydrochloride(Clinimycin HCl) is used as a solid in capsules. |
| Kinase Assay | In vitro potency assays: After RO4929097 is used, the Aβ peptides are measured by ECL assays using a variety of anti-Aβ antibodies and an Origen 1.5 Analyzer. The 4 g8 murine mAb binds an epitope in the Aβ peptide (within amino acids 18–21) that is immediately distal to the α-secretase cleavage site. The G2–10 murine mAb binds the C terminus that is exposed after γ-secretase-mediated cleavage to generate amino acid 40 of the Aβ40 peptide. The FCA3542 rabbit antibody binds the C terminus that is exposed after γ-secretase-mediated cleavage to generate amino acid 42 of the Aβ42 peptide. The 4 g8 mAb is biotinylated with biotin-LC-sulfo-N-hydroxysuccinimide-ester. The G2–10 and FCA3542 antibodies are ruthenylated with TAG-N-hydroxysuccinimide ester. Aβ(x-40) is detected with biotinylated 4 g8 and ruthenylated G2–10. Aβ(x-42) is detected with biotinylated 4 g8 and ruthenylated FCA3542. |
| In vitro | Clindamycin is a classical inhibitor of bacterial protein synthesis which binds to the 23S ribosomal RNA of the 50S ribosomal subunit. [1] |
| In vivo | Clindamycin hydrochloride is rapidly absorbed orally in dogs, exhibiting a mean absorption time (MAT) of 0.87 hours and a bioavailability of 72.55%. It shows a total clearance (CL) rate post intravenous (IV) and oral administration of 0.503 and 0.458 L/h/kg respectively, and reaches a steady-state volume of distribution (IV) of 2.48 L/kg, indicating extensive distribution throughout the body's fluids and tissues. Serum concentrations of clindamycin remain above 0.5 μg/mL for approximately 10 hours after both IV and oral administration. [1] Additionally, it significantly reduces oral malodor, dental plaque, dental calculus, and gingival bleeding in dogs over a period of 42 days. [2] At a dosage of 2.5 mg/lb following ultrasonic scaling, root planing, and polishing (USRP), clindamycin significantly impacts plaque and pocket depth related to periodontal disease, though not gingivitis. [3] Furthermore, it achieves a complete remission ratio of 71.4% (15/21) in dogs with canine superficial bacterial pyoderma within 14 to 28 days. [4] |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| Solubility Information | 10% DMSO+40% PEG300+5% Tween 80+45% Saline : 2 mg/mL (4.33 mM), Sonication is recommended.
DMSO : 75 mg/mL (162.53 mM), Sonication is recommended.
|
| Keywords | Inhibitor | inhibit | Clindamycin hydrochloride | Clindamycin Hydrochloride | Clindamycin | Bacterial | Antibiotic |
| Inhibitors Related | Neomycin sulfate | Dehydroacetic acid sodium | Ampicillin sodium | Methyl anthranilate | Doxycycline (hyclate) | Kanamycin sulfate | Urethane | Sulfamethoxazole sodium | Doxycycline | EDTA copper(II) disodium salt | Isoeugenol | Dimethyl sulfoxide |
| Related Compound Libraries | FDA-Approved & Pharmacopeia Drug Library | Bioactive Compound Library | Drug Repurposing Compound Library | Inhibitor Library | NO PAINS Compound Library | Anti-Bacterial Compound Library | FDA-Approved Drug Library | Orally Active Compound Library | Immunology/Inflammation Compound Library | Clinical Compound Library | Bioactive Compounds Library Max | Anti-Infection Compound Library |

 United States
United States