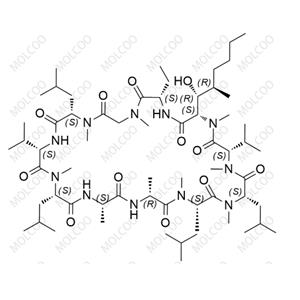
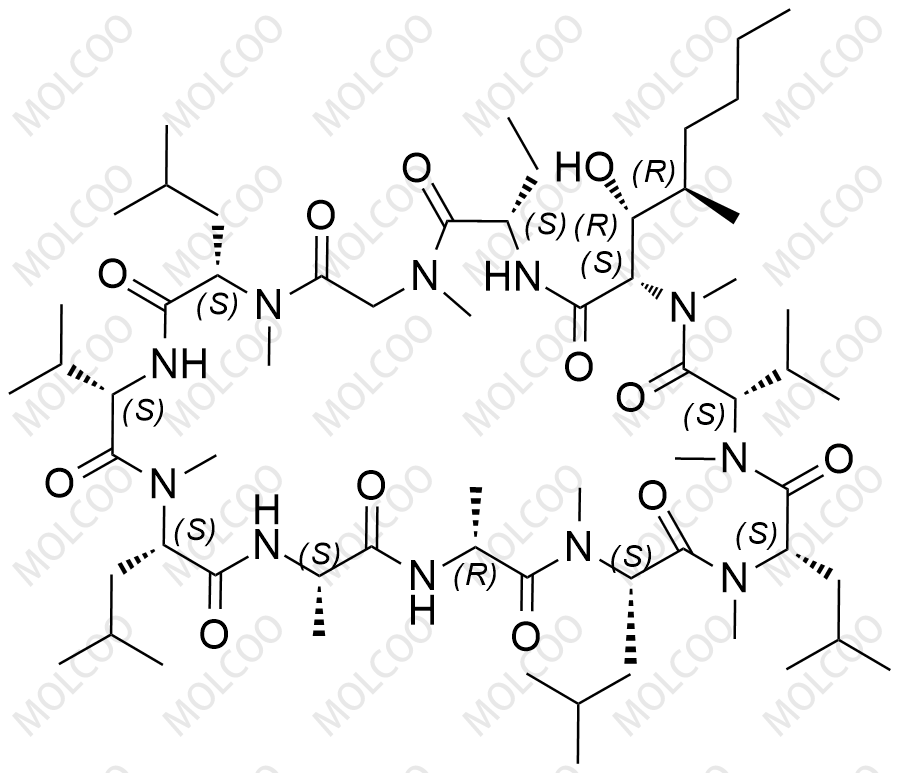
Ciclosporin EP Impurity B
Cyclosporine Impurity Reference Standards
Cyclosporine impurity reference standards are crucial for drug development and quality control. Cyclosporine is a widely used immunosuppressant for preventing rejection reactions in allogeneic organ transplantations. To ensure the safety and efficacy of the drug, accurate detection and quantitative analysis of cyclosporine and its impurities are essential.
Our cyclosporine impurity reference standards cover a variety of cyclosporine impurities, including but not limited to Cyclosporine Impurity A, B, C, D, and more. These impurity reference standards have undergone rigorous quality control and purity testing to ensure they meet international and industry standards.
With cyclosporine impurity reference standards, you can:
Accurate Identification: By comparing with impurity reference standards, accurately identify impurity components in the drug.
Quantitative Analysis: Utilize impurity reference standards for quantitative analysis to ensure that the impurity content in the drug is within an acceptable range.
Quality Control: Use impurity reference standards for quality control during drug production and development to ensure product quality is stable and reliable.
We are committed to providing high-quality cyclosporine impurity reference standards to meet our customers' needs in drug development and quality control.


 China
China