| Product Name | Chromium |
| CAS No | 7440-47-3 |
| EC-No | 231-157-5 |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 50000KG/month |
| Release date | 2025/03/21 |
| CAS: | 7440-47-3 |
| MF: | Cr |
| MW: | 52 |
| EINECS: | 231-157-5 |
| Product Categories: | Reagent Grade;Metals;Inorganics;Catalysis and Inorganic Chemistry;Chemical Synthesis;Chromium;ChromiumMetal and Ceramic Science;Metal and Ceramic Science;ChromiumEssential Chemicals;Reagent Plus;Routine Reagents;metal or element |
| Mol File: | 7440-47-3.mol |
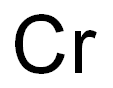 | |
| Chromium Chemical Properties |
| Melting point | 1857 °C (lit.) |
| Boiling point | 2672 °C (lit.) |
| density | 7.14 g/mL at 25 °C (lit.) |
| Fp | 50 °F |
| storage temp. | no restrictions. |
| form | powder |
| Specific Gravity | 7.2 |
| color | Silver-gray |
| Odor | Odorless |
| PH | <1 (H2O, 20°C) |
| resistivity | 12.7 μΩ-cm, 20°C |
| Water Solubility | Insoluble in water. |
| Merck | 13,2252 |
| Exposure limits | TLV-TWA: chromium metal 0.5 mg/m3 (ACGIH and MSHA), 1 mg/m3 (OSHA); Cr(II) and Cr(III) compounds 0.5 mg/m3 (ACGIH); Cr(VI) compounds, water soluble and certain water insoluble, 0.05 mg/m3 (ACGIH). |
| Stability: | Stable. Incompatible with carbonates, strong bases, mineral acids, lithium, sulfur dioxide, strong acids. |
| CAS DataBase Reference | 7440-47-3(CAS DataBase Reference) |
| IARC | 3 (Vol. Sup 7, 49) 1990 |
| NIST Chemistry Reference | Chromium(7440-47-3) |
| EPA Substance Registry System | Chromium (7440-47-3) |
| Description | Chromium as a metallic element was first discovered over 200 years ago, in 1797. But the history of chromium really began several decades before this. In 1761, in the Beresof Mines of the Ural Mountains, Johann Gottlob Lehmann obtained samples of an orange-red mineral, which he called ‘Siberian red lead.’ He analyzed this mineral in 1766 and discovered that it contained lead “mineralized with a selenitic spar and iron particles.” The mineral he found was crocoite, a lead chromate (PbCrO4). |
| Chemical Properties | Chromium may exist in one of three valence states in compounds, , , and . The most stable oxidation state is trivalent chromium; Hexavalent chromium is a less stable state. Chromium (element) blue-white to steel-gray, lustrous, brittle, hard, odorless solid. Elemental: |
| Physical properties | Chromium is a silvery white/gray, hard, brittle noncorrosive metal that has chemical andphysical properties similar to the two preceding elements in period 4 (V and Ti). As one of thetransition elements, its uses its M shell rather than its outer N shell for valence electrons whencombining with other elements. Its melting point is 1,857°C, its boiling point is 2,672°C,and its density is 7.19 g/cm3. |
| Isotopes | There are 26 isotopes of the element chromium; four are stable and foundin nature, and the rest are artificially produced with half-lives from a few microsecondsto a few days. The four stable isotopes and their percentage of contribution to thetotal amount of chromium on Earth are as follows: 50Cr = 4.345%, 52Cr = 83.789%,53Cr = 9.501%, and 54Cr = 2.365%. Cr-50 is radioactive but has such a long halflife—1.8×10+17 years—that it is considered to contribute about 4% to the total amount ofchromium found on Earth. |
| Origin of Name | From the Greek word chroma or chromos, meaning “color,” because of the many colors of its minerals and compounds. |
| Occurrence | Chromium is the 21st most common element found in the Earth’s crust, and chromiumoxide (Cr2O3) is the 10th most abundant of the oxide compounds found on Earth. It is notfound in a free metallic state.The first source of chromium was found in the mineral crocoite. Today it is obtained fromthe mineral chromite (FeCr2O4), which is found in Cuba, Zimbabwe, South Africa, Turkey,Russia, and the Philippines. Chromite is an ordinary blackish substance that was ignored formany years. There are different grades and forms of chromium ores and compounds, based onthe classification of use of the element. Most oxides of chromium are found mixed with othermetals, such as iron, magnesium, or aluminum.Astronauts found that the moon’s basalt rocks contain several times more chromium thanis found in basalt rocks of Earth. |
| Characteristics | Chromium is a hard, brittle metal that, with difficulty, can be forged, rolled, and drawn,unless it is in a very pure form, in which case the chromium is easier to work with. It is anexcellent alloying metal with iron. Its bright, silvery property makes it an appropriate metal toprovide a reflective, non-corrosive attractive finish for electroplating.Various compounds of chromium exhibit vivid colors, such as red, chrome green, andchromate yellow, all used as pigments. |
| History | Chromium was discovered in 1797 by Vauquelin, who prepared the metal the next year, chromium is a steel-gray, lustrous, hard metal that takes a high polish. The principal ore is chromite (FeCr2O4), which is found in Zimbabwe, Russia, South Africa, Turkey, Iran, Albania, Finland, Democratic Republic of Madagascar, the Philippines, and elsewhere. The U.S. has no appreciable chromite ore reserves. Chromium is usually produced by reducing the oxide with aluminum. Chromium is used to harden steel, to manufacture stainless steel, and to form many useful alloys. Much is used in plating to produce a hard, beautiful surface and to prevent corrosion. Chromium is used to give glass an emerald green color. It finds wide use as a catalyst. All compounds of chromium are colored; the most important are the chromates of sodium and potassium (K2CrO4) and the dichromates (K2Cr2O7) and the potassium and ammonium chrome alums, as KCr(SO4)2·12H2O. The dichromates are used as oxidizing agents in quantitative analysis, also in tanning leather. Other compounds are of industrial value; lead chromate is chrome yellow, a valued pigment. Chromium compounds are used in the textile industry as mordants, and by the aircraft and other industries for anodizing aluminum. The refractory industry has found chromite useful for forming bricks and shapes, as it has a high melting point, moderate thermal expansion, and stability of crystalline structure. Chromium is an essential trace element for human health. Many chromium compounds, however, are acutely or chronically toxic, and some are carcinogenic. They should be handled with proper safeguards. Natural chromium contains four isotopes. Twenty other isotopes are known. Chromium metal (99.95%) costs about $1000/kg. Commercial grade chromium (99%) costs about $75/kg. |
| Uses | In manufacture of chrome-steel or chrome-nickel-steel alloys (stainless steel), nonferrous alloys, heat resistant bricks for refractory furnaces. To greatly increase strength, hardness and resistance of metals to abrasion, corrosion and oxidation. For chrome plating of other metals; leather tanning; as pigment and mordant; wood preservative. Use of 51Cr as diagnostic aid see sodium chromate(VI). |
| Uses | Chromium is used in the manufacture ofits alloys, such as chrome-steel or chromenickel-steel. It is also used for chromeplatingof other metals, for tanning leather,and in catalysts. It occurs in chromite ores(FeO·Cr2O3). |
| Uses | The best-known use of chromium is for the plating of metal and plastic parts to producea shiny, reflective finish on automobile trim, household appliances, and other items where abright finish is considered attractive. It also protects iron and steel from corrosion.It is used to make alloys, especially stainless steel for cookware, and items for whichstrength and protection from rusting and high heat are important.Its compounds are used for high-temperature electrical equipment, for tanning leather, asa mordant (fixes the dyes in textiles so that they will not run), and as an antichalking agentfor paints.Some research has shown that, even though most chromium compounds are toxic, a smalltrace of chromium is important for a healthy diet for humans. A deficiency produces diabeteslike symptoms, which can be treated with a diet of whole-grain cereal, liver, and brewer’s yeast.Chromium’s most important radioisotope is chromium-51, which has a half-life of about27 days. It is used as a radioisotope tracer to check the rate of blood flowing in constrictedarteries.Some chromium compounds (e.g., chromium chloride, chromic hydroxide, chromic phosphate) are used as catalysts for organic chemical reactions.In 1960 the first ruby laser was made from a ruby crystal of aluminum oxide (Al2O3). Thesecrystals contain only a small amount of chromium, which stores the energy and is responsiblefor the laser action. A small amount of chromium found in the mineral corundum is responsible for the bright red color of the ruby gemstone. |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock

Company Profile Introduction
-








