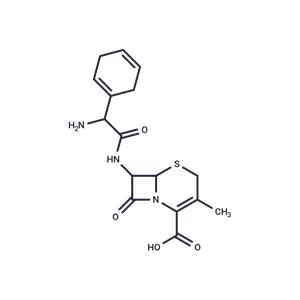
| Name | Cephradine |
| Description | Cephradine (Anspor) is a beta-lactam, first-generation cephalosporin antibiotic with bactericidal activity. |
| Cell Research | Cells Are seeded in six-well culture plates (9.6 cm2, growth area) at a density of 105 cells/cm2. At 2 days postseeding, the medium is removed and cells are washed twice with pH 6.0 uptake buffer. Independent studies are performed for each drug solution at 1 mg/mL made in pH 6.0 uptake buffer. At each time point (10, 20, 30, 45, 60, 90 min), drug solution is removed and cells are washed three times with ice-cold pH 6.0 uptake buffer. One milliliter of Milli-Q water is added to each well and incubated for 30 min at 25 °C. Cells are harvested and sonicated for 1-2 min. ZnSO4 solution (8%, 200 mL) is added to the cell lysate, vortexed rigorously, and centrifuged for 5 min at 3000 rpm. After filtration of the supernatant through a membrane filter (0.45 mm), samples are analyzed by HPLC. (Only for Reference) |
| In vivo | Cephradine has a low order of acute, subacute, and chronic oral toxicity, and acute and subacute parenteral toxicity. After daily intravenous doses of cephradine has been given to monkeys for 2 weeks, these animals show no signs of nephrotoxicity. Cephradine does not induce any teratogenic changes in the offspring of either mice or rats. The route of administration has been a determinant in the choice of a cephalosporin antibiotic. It is a distinct therapeutic advantage that a single cephalosporin antibiotic, cephradine, may be used in both oral and parenteral preparations[2]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | H2O : 8.33 mg/mL (23.84 mM), Sonication is recommended.
DMSO : 3.6 mg/mL (10.30 mM)
|
| Keywords | Gram-negative | Cephradine | Grampositive | Antibiotic | Oral | TOPK | SQ11436 | Genitourinary | Gastrointestinal | Bacterial | Infections | Inhibitor | SQ 11436 | inhibit | Cefradine |
| Inhibitors Related | Neomycin sulfate | Dehydroacetic acid sodium | Ampicillin sodium | Methyl anthranilate | Kanamycin sulfate | G-418 disulfate | Sulfamethoxazole sodium | Metronidazole | Doxycycline | EDTA copper(II) disodium salt | Dimethyl sulfoxide | Crystal Violet |
| Related Compound Libraries | FDA-Approved & Pharmacopeia Drug Library | Bioactive Compound Library | Pain-Related Compound Library | Approved Drug Library | Kinase Inhibitor Library | Drug Repurposing Compound Library | FDA-Approved Kinase Inhibitor Library | FDA-Approved Drug Library | Bioactive Compounds Library Max |

 United States
United States