| Description | Celite mainly refers to diatomaceous earth. It is a naturally occurring, soft, siliceous sedimentary rock. It is consists of fossilized remains of a type of hard-shelled algae, diatoms. It contains mostly silica (80% to 90%) as well as small amount of alumina and iron oxide. Celite has various applications. It can be supplied to the explosive to make them more stable for transport. It can also be used as a filtration acid as well as abrasive to be used in toothpaste, metal polishes as well as some facial scrubs. It can also be used as an insecticide for pest control. Its thermal properties make it be used as the barrier material in some fire resistant safes. It can also be used as a liquid absorbent, matting agent of coatings, catalyst support, activator in blood clotting studies, stabilizing component of dynamites, and a thermal insulator. It is also useful in construction. |
| References | https://en.wikipedia.org/wiki/Diatomaceous_earth |
| Chemical Properties | tan powder |
| Chemical Properties | Diatomaceous earth is a transparent to gray, odorless amorphous powder. |
| Chemical Properties | Amorphous silica, the noncrystalline form of SiO2, is a transparent to gray, odorless, amorphous powder |
| Uses | Chemically, diatomaceous earth is mostly amorphous silicon dioxide, accompanied by mineral salts, with large surface area. Its use as an insecticide dust is based on its capability to absorb moisture, oils, and waxes (48). |
| Uses | Filter Aid, Celite AW Standard Supercel? is used for general filtrations. It is also used as a functional filler in paints, plastics, paper, rubber, adhesives, catalysts, agricultural chemicals, pharmaceuticals and other chemicals, and a mild abrasive in polishes and cleaners. It is also used in clarifying liquids and oils, as an efficient thermal insulator; in manufacture of heat insulators, fire brick, and fire- and acid-proof packing materials; as an absorbent; in absorbent dynamite; as a solid carrier in chromatography; pozzolanic admixture in concrete mixes. |
| Uses | Highly insoluble, inert material for use as a filtration aid. |
| Definition | Diatomite: A whitish powdered mineral consisting mainly of silica (silicon( IV) oxide) derived from the shells of diatoms. It is used to make fireproof cements and as an absorbent in the manufacture of DYNAMITE. |
| General Description | Celpure? P65 (Diatomaceous earth, DE) is natural amorphous silica formed from the fossilized skeletons of diatoms. It is extracted, crushed, dried and calcined with or without flux material. DE is widely used as filler in construction materials, paints and as an anti-caking agent for agricultural chemicals. It has high water and oil absorption ability. The potential toxicity of different samples of DE has been assessed. DE is also employed as an adsorbent for column chromatography. |
| Agricultural Uses | Diatomaceous earth: Diatomaceous earth kills and repels insects, including bed bugs, fleas, cockroaches, and carpet beetles, by dehydrating them, rather than chemically poisoning them, and has been used since early times. It is also used as a filtering agent and as a filler in construction materials, pesticides, paints, and varnishes. The calcined version (which has been heat treated) is the most dangerous and contains crystallized silica, and should be handled as silica. |
| Trade name | CELITE®; CROP GUARD®; DIACTIV®; DIAFIL®; DI-ATOMATE®; DIE-SECTICIDE®; KENITE®; PRIMISIL® |
| Potential Exposure | Diatomaceous earth is used as a filtering agent and as a filler in construction materials, pesticides, paints, and varnishes. The calcined version (which has been heat treated) is the most dangerous and contains crystallized silica, and should be handled as silica. See also other entries on silica |
| Shipping | This material is not singled out by DOT in its Performance-Oriented Packaging Standards. |
| Incompatibilities | Silica, amorphous is a noncombustible solid. Generally unreactive chemically. Incompatible with fluorine, oxygen difluoride, chlorine trifluoride. Soluble in molten alkalis and reacts with most metallic oxides at high temperature. |
| Waste Disposal | Sanitary landfill. |









 China
China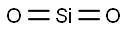

 Company information
Company information Advantage
Advantage


