Manufacturer Provide High Purity Ceftiofur Hydrochloride CAS 103980-44-5

Introduction
1.Product Name:Ceftiofur Hydrochloride
2.CAS NO: 103980-44-5
3.Purity:99%
4.Appearance:White to off white powder
5.Molecular formula: C19H18ClN5O7S3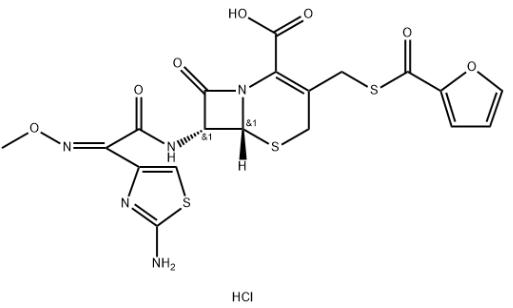
6.Molecular weight :560.01
7.Melting Point :>190°C(lit.)
8.Solubility : Soluble in DMSO (a little), methanol (a little)
9.Storage :Keep in a cool,dry,dark location in a tightly sealed container or cylinder.
Sodium ceftiofur is mainly used for bacteria and salmonella in severe infections and poultry.
1, viruses, bacteria, systemic or local infection caused by high temperature high fever, cough, gasping, difficulty breathing, skin and red blue and a purple color around the ears and mouth hoof canker, abortion, stillbirth, circle, posterior paresis, don't eat, can't afford to lay down, constipation and diarrhea, etc.
2. Infectious pleurisy, actinobacterium, bacilli, streptococcus mucoccus, piglets, yellow and white dysentery, salmonella, paracylobacter, atrophic rhinitis, chlamydia, etc.
3. High fever, endometritis, mastitis, milk-free milk syndrome, hoof leaf inflammation, hoof fever, cattle and sheep pasteuritis, transport fever, pneumonia, nasal atrophy, etc.
| ITEMS | SPECIFICATIONS | RESULTS |
| Appearance | White to Off-white Crystalline powder | Conform |
| Identification | The retention time of the principal peak in the
chromatogram obtained with the test solution
correspoonds to that of the principal peak in the
chromatogram obtained with reference solution. | Conform |
| Reaction of Chloride | Conform |
| Appearance of solution | Clarity | Not stronger than No.1 Turbidity Standard | <1 |
| Color | Not darker than standard solution Y9 | <8 |
| Solubility | Soluble in N, N- Dimethylacetamide, slightly
soluble in 5% of sodium bicarbonate and methanol. | Conform |
| PH | 2.0~3.0 | 2.2 |
| Water Content | 1.5%~4.0% | 1.6% |
| Specific optical Rotation | -115°~-127° | -117° |
| Heavy metal | NMT 0.0020% | Conform |
| Residue of ignition | NMT 0.20% | 0.15% |
| Related substances | The biggest impurity | NMT 0.20% | 0.09 |
| Total impurities | NMT 1.50% | 0.38 |
| Residue solvents | Ethyl Acetate | NMT 0.5000% | 0 |
| THF | NMT 0.0720% | 0.006 |
| Acetone | NMT 0.5000% | 0.2739% |
| Benzene | NMT 0.0002% | 0.0001 |
| Assay | Ceftiofur Hydrochloride | NLT 98.0% | 100.4% |
| Chloride | 6.0%~6.5% | 6.2% |
| Bacterial Endotoxin | Less than 0.20EU/mg | Conform |
| Sterility | Conforms the test | Conform |
| Particle size | D (0,1) | <1 | 1.8 |
| D (0,5) | <4 | 1.4 |
| D (0,9) | <7 | 3.7 |
| D (1,0) | <10 | 4.2 |
| CONCLUSION | THE TEST RESULTS CONFORMS TO THE SPECIFICATION |







 China
China




