Product Code: C054024
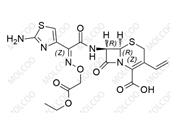
English Name: Cefixime Impurity 24
English Alias: (6R,7R)-8-oxo-7-(2-phenylacetamido)-3-vinyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
CAS No.:33748-00-4
Molecular Formula: C₁₇H₁₆N₂O₄S
Molecular Weight: 344.38
High-Purity Reference Standard: As a reference standard for Cefixime Impurity 24, its structure is confirmed by NMR and MS with ≥98.5% purity (HPLC), suitable for impurity analysis and quality control of cephalosporin antibiotics.
Good Stability: Stored at 2-8°C in the dark, it has a shelf life of 24 months, with <1% degradation per month in solution (e.g., methanol), ensuring reliable detection results.
Antibiotic Impurity Detection: Used for HPLC/LC-MS detection of Impurity 24 in Cefixime API and formulations, controlling impurity content ≤0.2% (refer to ICH Q3A).
Process Optimization: In Cefixime synthesis, monitor the generation of this impurity during the acylation stage (e.g., generation increases 3-fold at temperatures >50°C), and reduce its content to below 0.1% by adjusting reaction pH (controlled at 7.0-7.5).
Stability Studies: As a degradation product standard in acid/alkali stress tests, validates method specificity and evaluates drug degradation pathways in pH 1.2/6.8 buffers.
Cefixime, a third-generation cephalosporin antibiotic, is used to treat bacterial infections. Impurity 24 may arise from side chain introduction or cyclization by-products during its synthesis. This impurity's structure contains vinyl and phenylacetyl groups, potentially affecting drug antibacterial activity and safety. FDA guidelines for cephalosporin impurities require genotoxicity screening for such β-lactam impurities, driving the use of high-purity reference standards.
Detection Technology: UPLC-MS/MS is used with a C18 column (1.7μm, 2.1×100mm) and 0.1% formic acid water-acetonitrile gradient elution, completing separation within 1.5 minutes with a detection limit of 0.01 ng/mL, 4 times more efficient than traditional HPLC.
Formation Mechanism: Mainly originates from isomerization side reactions of the vinyl side chain during the acylation of 7-aminocephalosporanic acid (7-ACA) with phenylacetyl chloride. Using triethylamine as a base reduces isomerization, decreasing impurity content from 0.6% to 0.03%.
Safety Evaluation: In vitro experiments show the impurity has an MIC of 8 μg/mL against E. coli (Cefixime API is 0.125 μg/mL), indicating significantly reduced antibacterial activity but potential allergenicity. EMA sets its permissible daily exposure (PDE) ≤5 μg.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!






 China
China