1/5
Caprylic/capric triglyceride NEW
- Min. Order1KG
- Purity99%
- Cas No73398-61-5
- Supply Ability50000KG/month
- Update time2023-09-07

| Product Name | Caprylic/capric triglyceride |
| CAS No | 73398-61-5 |
| EC-No | 277-452-2 |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 50000KG/month |
| Release date | 2023/09/07 |
| CAS: | 73398-61-5 |
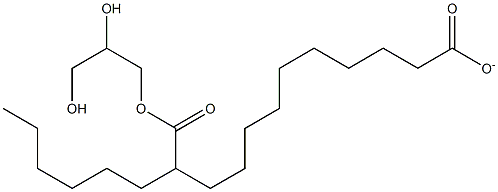
| MF: | C21H39O6- |
| MW: | 387.53076 |
| EINECS: | 277-452-2 |
| Product Categories: | |
| Mol File: | 73398-61-5.mol |
 | |
| Caprylic/capric triglyceride Chemical Properties |
| density | 0.94-0.96 |
| vapor pressure | 0-0Pa at 20℃ |
| solubility | Soluble in all proportions at 20°C in acetone, benzene, 2-butanone, carbon tetrachloride, chloroform, dichloromethane, ethanol, ethanol (95%), ether, ethyl acetate, petroleum ether, special petroleum spirit (boiling range 80–110°C), propan- 2-ol, toluene, and xylene. Miscible with long-chain hydrocarbons and triglycerides; practically insoluble in water. |
| form | Liquid |
| InChI | InChI=1S/C21H40O6/c1-2-3-4-10-13-18(21(26)27-17-19(23)16-22)14-11-8-6-5-7-9-12-15-20(24)25/h18-19,22-23H,2-17H2,1H3,(H,24,25)/p-1 |
| InChIKey | YWHITOKQSMJXEA-UHFFFAOYSA-M |
| SMILES | C([O-])(=O)CCCCCCCCCC(C(OCC(O)CO)=O)CCCCCC |
| LogP | 8.2-10.9 |
| EPA Substance Registry System | Mixed decanoyl and octanoyl glycerides (73398-61-5) |
| Caprylic/capric triglyceride Usage And Synthesis |
| Description | Caprylic/capric triglyceride is a natural coconut and palm kernel oil derived mixture ofglycerin triester with caprylic and capric acids, which can be used as an alternative to mineral and vegetable oils. It is a mixed ester composed of caprylic and capric fatty acids attached to a glycerin backbone. It is an excellent moisturising emollient oil with good skin care properties, giving a pleasant non-slippery after feel on the skin. It is used as a less oily alternative to castor oil in lipsticks and in colour cosmetics.? |
| Chemical Properties | A colorless to slightly yellowish oily liquid that is practically odorless and tasteless. It solidifies at about 0°C. The oil is free from catalytic residues or the products of cracking. Caprylic/capric triglyceride containing the tri-glycerides of medium chain, saturated fatty acids mainly C-8 (Caprylic) and C-10 (Capric). |
| Production Methods | Medium-chain triglycerides are obtained from the fixed oil extracted from the hard, dried fraction of the endosperm of Cocos nucifera L. Hydrolysis of the fixed oil followed by distillation yields the required fatty acids, which are then re-esterified to produce the medium-chain triglycerides. Although the PhEur 6.0 specifies that medium-chain fatty acids are obtained from coconut oil, medium-chain triglycerides are also to be found in substantial amounts in the kernel oils of certain other types of palm-tree, e.g. palm kernel oil and babassu oil. Some animal products, such as milk-fat, also contain small amounts (up to 4%) of the medium-chain fatty acid esters. |
| Pharmaceutical Applications | Medium-chain triglycerides have been used in a variety of pharmaceutical formulations including oral, parenteral, and topical preparations. In oral formulations, medium-chain triglycerides are used as the base for the preparation of oral emulsions, microemulsions, selfemulsifying systems, solutions, or suspensions of drugs that are unstable or insoluble in aqueous media, e.g. calciferol. Mediumchain triglycerides have also been investigated as intestinalabsorption enhancersand have additionally been used as a filler in capsules and sugar-coated tablets, and as a lubricant or antiadhesion agent in tablets. In parenteral formulations, medium-chain triglycerides have similarly been used in the production of emulsions, solutions, or suspensions intended for intravenous administration.In rectal formulations, medium-chain triglycerides have been used in the preparation of suppositories containing labile materials. In cosmeticsand topical pharmaceutical preparations, medium-chain triglycerides are used as a component of ointments, creams, and liquid emulsions. Therapeutically, medium-chain triglycerides have been used as nutritional agents.Diets containing medium-chain triglycerides are used in conditions associated with the malabsorption of fat, such as cystic fibrosis, since medium-chain triglycerides are more readily digested than long-chain triglycerides. Medium-chain triglycerides have been particularly investigated for their use in total parenteral nutrition (TPN) regimens in combination with longchain triglycerides. Although similar to long-chain triglycerides, medium-chain triglycerides have a number of advantages in pharmaceutical formulations, which include better spreading properties on the skin; no impedance of skin respiration; good penetration properties; good emollient and cosmetic properties; no visible film on the skin surface; good compatibility; good solvent properties; and good stability against oxidation. |
| Safety | Medium-chain triglycerides are used in a variety of pharmaceutical formulations including oral, parenteral, and topical products, and are generally regarded as essentially nontoxic and nonirritant materials. In acute toxicology studies in animals and humans, no irritant or other adverse reactions have been observed; for example, when they were patch-tested on more than 100 individuals, no irritation was produced on either healthy or eczematous skin. Medium-chain triglycerides are not irritating to the eyes. Similarly, chronic toxicology studies in animals have shown no harmful adverse effects associated with medium-chain triglycerides following inhalation or intraperitoneal, oral, and parenteral administration. In humans, administration of 0.5 g/kg body-weight mediumchain triglycerides to healthy individuals produced no change in blood or serum triglycerides compared to subjects receiving the same dose of the long-chain triglyceride triolein. In patients consuming diets based on medium-chain triglycerides, adverse effects reported include abdominal pain and diarrhea. LD50 (mouse, IV): 3.7 g/kg LD50 (mouse, oral): 29.6 g/kg LD50 (rat, oral): 33.3 g/kg |
| storage | storage temperatures that can be experienced in tropical and temperate climates. Ideally, however, they should be stored at temperatures not exceeding 25°C and not exposed to temperatures above 40°C for long periods. At low temperatures, samples of medium-chain triglycerides may become viscous or solidify. Samples should therefore be well melted and mixed before use, although overheating should be avoided. In the preparation of microemulsions and self-emulsifying systems, emulsions, or aqueous suspensions of medium-chain triglycerides, care should be taken to avoid microbiological contamination of the preparation, since lipase-producing microorganisms, which become active in the presence of moisture, can cause hydrolysis of the triglycerides. Hydrolysis of the triglycerides is revealed by the characteristic unpleasant odor of free mediumchain fatty acids. Medium-chain triglycerides may be sterilized by maintaining at 170°C for 1 hour. Medium-chain triglycerides should be stored protected from light in a well-filled and well-closed container. When stored dry, in sealed containers, medium-chain triglycerides remain stable for many years. |
| Incompatibilities | Preparations containing medium-chain triglycerides should not come into contact with polystyrene containers or packaging components since the plastic rapidly becomes brittle upon contact. Low-density polyethylene should also not be used as a packaging material as the medium-chain triglycerides readily penetrate the plastic, especially at high temperatures, forming an oily film on the outside. High-density polyethylene is a suitable packaging material. Closures based on phenol resins should be tested before use for compatibility with medium-chain triglycerides. Polyvinyl chloride packaging should also be tested for compatibility since mediumchain triglycerides can dissolve some plasticizers, such as phthalates, out of the plastic. Materials recommended as safe for packaging medium-chain triglycerides are low-density polyethylene, polypropylene, glass, and metal. |
| Regulatory Status | GRAS listed. Included in the FDA Inactive Ingredients Database (topical preparations). Included in nonparenteral and parenteral medicines licensed in Europe. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
-







