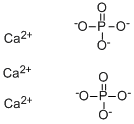
| Description | Calcium
Phosphate is the calcium salt of phosphoric acid with widely used
applications. It occur abundantly in nature in several forms and are the
principal minerals for the production of phosphate fertilizers and for a
range of phosphorus compounds. For example, the tribasic variety
(precipitated calcium phosphate), Ca3(PO4)2, is the principal inorganic
constituent of bone ash. The acid salt Ca(H2PO4)2, produced by treating
mineral phosphates with sulfuric acid, is employed as a plant food and
stabilizer for plastics. It is a natural constituent of mammals, and it
is a component of bone replacement transplants in much higher amounts
with no toxicological problems.Calcium phosphates are the largest group
of artificial bone graft substitutes. This is mainly due to their close
resemblance to the mineral components of bone. This Product can be used
as a countermeasure for exposure to strontium and radium radionuclides.
Upon oral uptake, calcium phosphate competes for and blocks the
absorption of radium (Ra-226) and strontium (Sr-90) in the
gastrointestinal (GI) tract. |
| Chemical Properties | white powder |
| Uses | Tribasic
calcium phosphate occurs in nature as minerals, oxydapatite,
whitlockite, voelicherite, apatite, phosphorite. It has many industrial
applications. Some are similar to the monobasic and dibasic salts. It is
used in fertilizers, dental products, ceramics and polishing powder.
Some other important applications are in plastics as a stabilizer; as an
anticaking agent; as a nutrient supplement in cattle food; for
clarifying sugar syrup; as a mordant in dyeing textiles; and as a buffer
to control pH. |
| Preparation | Tribasic
calcium phosphate is obtained from naturally occurring minerals for
fertilizer applications. The compound may be prepared in the laboratory
by the reaction of sodium phosphate with calcium chloride with excess of
ammonia. Also, it can be prepared by treatment of calcium hydroxide
with phosphoric acid:
2H3PO4 + 3Ca(OH)2 → Ca3(PO4)2 + 6H2O |
| Uses | manufacture
of fertilizers, H3PO4 and P Compounds; manufacture of milk-glass,
polishing and dental powders, porcelains, pottery; enameling; clarifying
sugar syrups; in animal feeds; as noncaking agent; in the textile
industry. |






 China
China