| Product Name | Calcium carbide |
| CAS No | 75-20-7 |
| EC-No | 200-848-3 |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 50000KG/month |
| Release date | 2025/02/28 |
| CAS: | 75-20-7 |
| MF: | C2Ca |
| MW: | 64.1 |
| EINECS: | 200-848-3 |
| Product Categories: | Carbides;Ceramics;Inorganics;Metal and Ceramic Science;Non metallic mineral;inorganic chemical raw material |
| Mol File: | 75-20-7.mol |
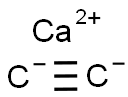 | |
| Calcium carbide Chemical Properties |
| Melting point | 447°C |
| Boiling point | 2300°C |
| density | 2.22 g/mL at 25 °C(lit.) |
| storage temp. | water-free area |
| form | pieces |
| color | Gray-black |
| Specific Gravity | 2.22 |
| Water Solubility | hydrolyses |
| Sensitive | Moisture Sensitive |
| Merck | 14,1656 |
| BRN | 3909011 |
| Exposure limits | ACGIH: TWA 2 mg/m3 OSHA: TWA 5 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 2 mg/m3 |
| Stability: | Stability Reacts violently with water liberating highly flammable gas (acetylene). Do not use water if this material is involved in a fire. Incompatible with moisture, water, strong oxidizing agents, alcohols, hydrogen chloride, magnesium. |
| InChIKey | UIXRSLJINYRGFQ-UHFFFAOYSA-N |
| EPA Substance Registry System | Calcium carbide (75-20-7) |
| Calcium carbide Usage And Synthesis |
| Description | Calcium carbide (molecule formula: CaC2), is a kind of important chemical raw materials produced from the chemical processing of limestone. In 1892, H. Maysan (French) and H. Wilson (United state) simultaneously developed a calcium carbide production approach based on furnace Reduction. The United State had successfully achieved industrial production in 1895. The property of calcium carbide is related to its purity. Its industrial product is mostly the mixture of calcium carbide and calcium oxide, and also contains trace amounts of sulfur, phosphorus, nitrogen and other impurities. With the increasing content of impurities, it color exhibits gray, brown to black. The melting point and electrical conductivity both decrease with the decrease of the purity. The purity of its industrial product is usually 80% with m.p. being 1800~2000 °C. At room temperature, it does not react with air, but it can have oxidation reaction at above 350 ℃, and have reaction with nitrogen at 600~700 ℃ to generate calcium cyanamide. Calcium carbide, when coming across with water or steam, generates acetylene and release a large amount of heating. CaC2 + 2H2O─ → C2H2 + Ca (OH) 2 + 125185.32J, 1kg of pure calcium carbide can produce 366 L of acetylene 366l (15 ℃, 0.1MPa). Thereby, for its storage: calcium carbide should be strictly kept away from water. It is usually packed in a sealed iron container, and sometimes stored in a dry warehouse being filled with nitrogen if necessary. |
| Uses | Industry Applications Benefit Chemical manufacture Production of acetylene gas Raw materials,CaC2 + 2 H2O → C2H2 + Ca(OH)2 Production of calcium cyanamide Raw materials, CaC2 + N2 → CaCN2 + C Production of various acetylene derivatives Source of acetylene gas Production of calcium hydroxide Raw materials, CaC2 + 2 H2O → C2H2 + Ca(OH)2 Steel production The desulfurisation of iron (pig iron, cast iron and steel) Desulfurization agent As a fuel in steelmaking Extend the scrap ratio to liquid iron Ladle treatment facilities A powerful deoxidizer Mining, automobiles and street lighting Carbide lamps React with water to make acetylene gas, which can burn to glow Fruit Artificial ripening fruit Source of acetylene gas Signal flares Floating, self-igniting naval signal flares Used together with calcium phosphide Cylinder gas Metal fabrication and construction Source of acetylene gas Experiment teaching Teaching reagent Experiment reagent |
| Reaction with water | Calcium carbide will immediately have reaction upon coming across with water, generating acetylene and calcium hydroxide, which is the approach of industrial preparation of acetylene (carbide method), the reaction equation is: CaC2 + 2H2O = C2H2 + Ca (OH) 2. Since the impurity of calcium carbide, the generated acetylene gas is usually mixed with a small amount of hydrogen sulfide, phosphine gas and other contaminants, so there is a bad smell. Calcium carbide is produced from the lime and coke in an electric furnace at a high temperature of 3000 ℃: 3C + CaO = CaC2 + CO. Upon the laboratory preparation of acetylene, owing to the reaction between calcium carbide and water is very fierce, we can apply saturated brine to substitute water so that a pure and smooth airflow of acetylene can be obtained. Calcium carbide won’t have reaction with sodium chloride. |
| Production method | Electric furnace reduction method is the only method for industrial production of calcium carbide at present. Put calcium oxide and coke for reduction reaction at 2000~2200 ℃: CaO + 3C─ → CaC2 + CO-480644.64J, the resulting molten calcium carbide flow into the receiver tank from the bottom of the reactor, and we obtain the final product after cooling. Calcium carbide production belongs to high temperature operation with relative large amount dust being produced and consuming a large amount of electrical energy. In 1980s, the production of per ton of calcium carbide consumes industrial power of about 10~11GJ. In order to reduce the power consumption, people mostly apply large-scale and closed calcium carbide furnace to reduce heat loss and also do good to the recycling of carbon monoxide. |
| Description | Calcium carbide,is a binary salt. It is a grayish-black hard solid that reacts with water to produce acetylene gas, a solid corrosive that is calcium hydroxide, and release heat. Acetylene gas is manufactured by reacting calcium carbide with water. Because acetylene is so unstable, it is not shipped in bulk quantities. Calcium carbide is shipped to acetylene-generating plants where it is reacted with water in a controlled reaction. After the reaction process, the acetylene gas is placed into specially designed containers with a honeycombed mesh inside for shipment and use. It is dissolved in acetone for stability. Calcium carbide has a specific gravity of 2.22, which is heavier than water. The four-digit UN identification number for calcium carbide is 1402. The NFPA 704 designation is health 3, flammability 3, and reactivity 2. The white section at the bottom of the diamond contains a W with a slash through it, indicating water reactivity. It is shipped in metal cans, drums, and specially designed covered bins on railcars and trucks. When shipped and stored, it should be kept in a cool, dry place. Primary uses are in the generation of acetylene gas for welding, vinyl acetate monomer, and as a reducing agent. |
| Chemical Properties | grey or black solid with a garlic-like odour |
| Physical properties | Grayish-black orthorhombic crystal; density 2.22 g/cm3; melts at 2,200°C; reacts with water. |
| Uses | Calcium carbide (CaC2) has a garlic-like odor and reacts with water to form acetylene gas plus calcium hydroxide and heat. In the past, it was used in miners’ lamps to continuously produce a small acetylene flame to provide some illumination in coal mines. |
| Uses | Calcium carbide is used as a desulfurizer, dehydrant of steel, fuel in steel making, powerful deoxidizer and as a source of acetylene gas. It is used as a starting material for the preparation of calcium cyanamide, ethylene, chloroprene rubber, acetic acid, dicyandiamide and cyanide acetate. It is used in carbide lamps, toy cannons such as the big-bang cannon and bamboo cannon. It is associated with calcium phosphide and used in floating, self-igniting naval signal flares. Further, it is involved in the reduction of copper sulfide to metallic copper. |
| Uses | Calcium carbide is the most relevant carbide industrially because of its important role as the basis of acetylene industry. In locations where there is shortage of petroleum, Calcium Carbide is used as the starting material for the production of acetylene (1 kg of carbide yields ~300 liters acetylene), which, in turn, can be used as a building block for a range of organic chemicals (e.g. vinyl acetate, acetaldehyde and acetic acid). In some locations, acetylene is also used to produce vinyl chloride, the raw material for the production of PVC. A less important use of Calcium Carbide is related to the ferilizers industry. It reacts with nitrogen to form calcium cyanamide, which is the starting material for the production of cyanamide (CH2N2). Cyanamide is a common agricultural product used to stimulate early foliation. Calcium Carbide can also be employed as desulfurizing agent for producing low-sulfur carbon steel. Also, it is used as a reducing agent to produce metals from their salts, e.g., for direct reduction of copper sulfide to metallic copper. |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
-








