Product Code: B012004
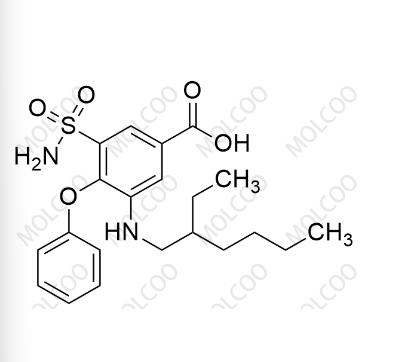
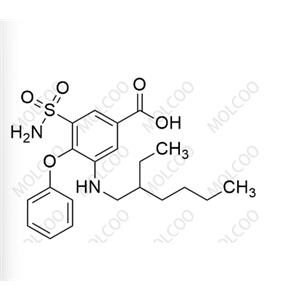
English Name: Bumetanide EP Impurity D
English Alias: 3-((2-ethylhexyl)amino)-4-phenoxy-5-sulfamoylbenzoic acid
CAS No.:153012-65-8
Molecular Formula: C₂₁H₂₈N₂O₅S
Molecular Weight: 420.52
High Purity Assurance: With a purity of ≥99.0% detected by HPLC, its structure is confirmed by NMR, HRMS and other techniques, providing a precise and reliable reference standard for the analysis of Bumetanide impurities.
Good Stability: Stored in a sealed and light - proof condition at 2 - 8°C, the shelf life can reach 24 months, and the degradation rate in solution (such as methanol) is less than 0.3% within 1 month, ensuring the stability and repeatability of experimental data.
Regulatory Compliance: Strictly produced in accordance with international pharmaceutical regulatory standards such as European Pharmacopoeia (EP), ICH and FDA, meeting the regulatory requirements of pharmaceutical companies in drug research and development, quality control and application processes.
Quality Control Testing: Used for the detection of Impurity D in Bumetanide active pharmaceutical ingredients and formulations by HPLC, LC - MS, etc., strictly controlling the impurity content to ensure that the product quality meets the European Pharmacopoeia and relevant regulatory standards.
Process Optimization: During the synthesis of Bumetanide, monitor the generation of Impurity D. Adjust process parameters such as reaction temperature, raw material ratio, and reaction time to reduce the amount of impurities generated and improve product purity.
Analytical Method Validation: As a reference standard for the development and validation of Bumetanide impurity detection methods, evaluate the specificity, sensitivity and repeatability of the methods, and provide reliable analytical means for quality control.
Stability Studies: Track the content changes of Impurity D in accelerated stability tests (such as 60℃/RH75%) and long - term stability tests, analyze its impact on drug stability, and provide data support for determining the storage conditions and shelf life of drugs.
Bumetanide is a potent loop diuretic commonly used in the treatment of edematous diseases, hypertension and other conditions. During its production, various impurities may be generated due to factors such as raw material purity, reaction conditions, and synthesis processes, and Impurity D is one of them. With the continuous improvement of global requirements for drug quality and safety, pharmaceutical regulatory agencies around the world have become increasingly strict in controlling impurities in Bumetanide, requiring comprehensive research on impurities and the establishment of strict limit standards. Therefore, the research and control of Bumetanide Impurity D have become an important part of ensuring drug quality and patient medication safety.
Detection Technology: Currently, UPLC - MS/MS technology is mainly used to detect Impurity D. By optimizing the chromatographic column (such as C18 column, 1.7μm particle size), mobile phase system (acetonitrile - water gradient elution with ammonium formate buffer salt) and mass spectrometry parameters (electrospray ionization source, multiple reaction monitoring mode), highly sensitive detection of impurities can be achieved, with a detection limit as low as 0.002 ng/mL.
Formation Mechanism: Research shows that Impurity D may originate from side reactions in the amination, phenoxy introduction or sulfonamidation steps during the synthesis of Bumetanide, or from the incomplete reaction and conversion of related intermediates in raw materials. By improving the synthesis process, such as optimizing catalyst selection and precisely controlling reaction conditions, the generation of Impurity D can be effectively reduced.
Safety Evaluation: The safety research on Impurity D is still in the preliminary stage. In vitro cell experiments show that it has a certain impact on some cell lines at high concentrations, but the specific toxic mechanism is still unclear. Currently, the limit set in drug quality standards is ≤0.1%. Further animal experiments and pre - clinical studies are needed to deeply explore its toxic characteristics and improve the impurity control strategy.





 China
China