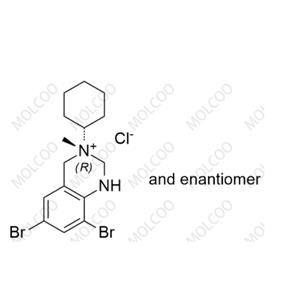
Product Number: B008006
English Name: Bromhexine Impurity E HCl
English Alias: (RS)-6,8-dibromo-3-cyclohexyl-3-methyl-1,2,3,4-tetrahydroquinazolin-3-ium chloride
CAS Number: 1660957-93-6
Molecular Formula: C₁₅H₂₁Br₂N₂·Cl
Molecular Weight: 424.6
As a hydrochloride impurity of bromhexine, the research advantages of this compound lie in:
Evaluating the by-product formation mechanism of halogenation reactions during bromhexine synthesis to optimize processes for controlling chiral and halogenated impurities;
Serving as a hydrochloride-form reference standard to precisely match the actual state of drugs, improving detection accuracy in impurity quantification;
Assisting in studying the potential impact of halogenated impurities and quaternary ammonium salt structures on drug safety to provide a basis for formulating reasonable impurity limits.
Drug Development: Used as an impurity reference standard to identify and quantify Impurity E HCl in bromhexine preparations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity and specificity of detection methods (e.g., HPLC or LC-MS), ensuring impurity content meets pharmacopoeia requirements during production;
Stability Studies: Simulating impurity formation pathways under drug storage conditions to assist in establishing storage conditions and shelf life.
Bromhexine, a commonly used mucolytic drug in clinical practice, is used for treating respiratory diseases. Due to the bromine atoms and nitrogen heterocycles in its molecular structure, halogenated by-products and stereoisomeric impurities are prone to generate during synthesis. Bromhexine Impurity E HCl, as an impurity containing dibromo substitution and quaternary ammonium salt structure, may be formed during bromination or salification. Its content directly affects drug quality, making research on this impurity an important part of the bromhexine quality control system.
Current research focuses on:
Synthesis Process: Developing high-purity synthesis methods for (RS) isomer hydrochloride to solve the challenge of chiral separation and obtain single-configuration reference standards;
Detection Technologies: Using chiral chromatography (e.g., HPLC with chiral columns) or ion chromatography for highly sensitive detection of this quaternary ammonium salt impurity;
Toxicological Evaluation: Studying the potential genotoxicity and cytotoxicity of bromo groups and quaternary ammonium structures through in vitro cytotoxicity assays and animal models;
Process Control: Analyzing the influence of bromination reaction conditions (e.g., temperature, catalyst) on impurity formation to optimize process parameters for reducing its content.





 China
China