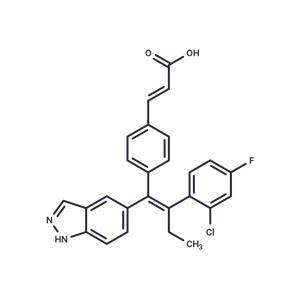
| Name | Brilanestrant |
| Description | Brilanestrant (GDC-0810) is a selective estrogen receptor degrader (IC50: 0.7 nM). |
| Cell Research | MCF-7 cells are adjusted to a concentration of 40000 cells per mL in RPMI containing 10% FBS and 20 mM HEPES. Then 16 μL of the cell suspension (640 cells) is added to each well of a 384-well plate, and the cells are incubated overnight to allow the cells to adhere. The following day a 10-point, serial 1:5 dilution of each compound is added to the cells in 16 μL at a final concentration ranging from 10 to 0.000005 μM. After 5 days' compound exposure, 16 μL of CellTiter-GLo is added to the cells, and the relative luminescence units of each well are determined. CellTiter-GLo added to 32 μL of medium without cells is used to obtain a background value. The percent viability of each sample is determined as follows: RLU sample-RLU background/RLU untreated cells-RLU background ×100=%viability. |
| Animal Research | Time release pellets containing 0.72 mg 17-β estradiol are subcutaneously implanted into nu/nu mice. MCF-7 cells are grown in RPMI containing 10% FBS at 5% CO2 37°C. Trypsinized cells are pelleted and resuspended in 50% RPMI(serum free)and 50% Matrigel at 1×107 cells/mL. MCF-7 cells are subcutaneously injected (100 μL/animal) on the right flank 2-3 days post pellet implantation. Tumor volume (length × width2/2) is monitored biweekly. When tumors reach an average volume of appr 200 mm3 animals are randomized and treatment is started. Animals are treated with vehicle or compound daily for 4 weeks. Tumor volume and body weight are monitored biweekly throughout the study. |
| In vitro | Brilanestrant is a ERα binder (IC50: 6.1 nM), a full transcriptional antagonist with no agonism (3× ERE, IC50: 2 nM), and displays good potency and efficacy in ER-α degradation (EC50: 0.7 nM) and MCF-7 breast cancer cell viability (IC50; 2.5 nM) assays [1]. Brilanestrant induces a distinct ERα conformation versus tamoxifen and other ER therapeutics, and does not exhibit tamoxifen-like ER agonism in MCF7 cells [2]. |
| In vivo | The pharmacokinetic profile of Brilanestrant shows it is a clearance molecule across species, with good bioavailability (40%-60%). Brilanestrant (3 mg/kg, p.o.) shows substantial tumor-growth inhibition in a tamoxifen-sensitive MCF-7 xenograft model, while at the highest dose of 100 mg/kg/day, all animals show tumor regression of more than 50% without weight loss [1]. Brilanestrant exhibits low clearance (11 mL/min/kg) and 61% oral bioavailability. Brilanestrant (1-100 mg/kg/day, p.o.) displays dose-dependent efficacy in the MCF7 xenograft model [2]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 55 mg/mL (123.07 mM)
H2O : Insoluble
|
| Keywords | inhibit | ARN810 | Estrogen Receptor/ERR | RG6046 | Inhibitor | Brilanestrant | RG-6046 | ARN 810 |
| Inhibitors Related | Kaempferol | Tamoxifen | Mifepristone | Estradiol | Astragaloside IV | Estradiol benzoate | Cholesterol | Melatonin | Chrysin | Allura Red AC | Natamycin | Ethisterone |
| Related Compound Libraries | Bioactive Compound Library | Anti-Cancer Clinical Compound Library | Drug Repurposing Compound Library | Endocrinology-Hormone Compound Library | Orally Active Compound Library | Clinical Compound Library | Bioactive Compounds Library Max | Anti-Cancer Compound Library | Anti-Cancer Active Compound Library | Anti-Cancer Drug Library |

 United States
United States