| Name | Betrixaban |
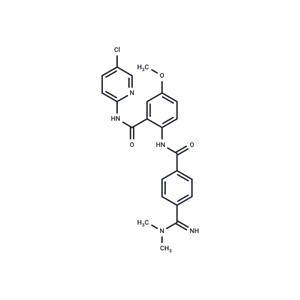
| Description | Betrixaban (PRT054021) is a non-vitamin K oral anticoagulant whose action is driven by the competitive and reversible inhibition of the factor Xa [1]. It was selected among all lead compounds due to its low hERG channel affinity while sustaining its factor Xa inhibition capacity [3]. Betrixaban, now developed by Portola Pharmaceuticals Inc., is prescribed as a venous thromboembolism (VTE) prophylactic for adult patients with moderate to severe restricted motility or with other risks for VTE [2]. VTE can be manifested as deep vein thrombosis or pulmonary embolism and it is a leading cause of preventable death in hospitalized patients [4]. |
| Kinase Assay | To measure the inhibition of fXa activity by direct fXa inhibitors and the reversal of its inhibitory effect by r-Antidote, purified human plasma fXa (3 nM) (Haematologic Technologies), varying concentrations of inhibitor (0, 2.5, 5.0 and 7.5 nM) and r-Antidote are added to the assay buffer (20 mM Tris, 150 mM NaCl, 5 mM Ca2+ and 0.1% BSA, pH 7.4). After incubation at room temperature for 30 min, 100 μM Spectrozyme-fXa is added to the mixture, and the initial rate of sub?strate cleavage is monitored continuously for 5 min at 405 nm in a 96-well plate reader. The initial velocity of product formation as a function of inhibitor and r-Antidote concentrations is analyzed by Dynafit to estimate the binding affinity of r-Antidote to each inhibitor[2]. . |
| Animal Research | Whole-blood INR values (mean±s.d.) in rats infused with Betrixaban (1 mg/kg per hour) or vehicle and then treated with either vehicle or r-Antidote by i.v. bolus (6 mg) over 5 min plus infusion (9 mg/h) for up to 90 min. Circles, vehicle+vehicle; squares, Betrixaban + vehicle; triangles, Betrixaban + r-Antidote. *P≤0.02 compared to the r-Antidote treatment group determined by unpaired two-tailed t test. Whole-blood INR values (mean±s.d.) in rats infused with Apixaban (0.5 mg per kg body weight h?1) or vehicle and then treated with either vehicle or r-Antidote by i.v. bolus (6 mg) over 5 min plus infusion (6 mg/h) for up to 90 min. Circles, vehicle + vehicle; squares, apixaban + vehicle; triangles, apixaban+r-Antidote. *P≤0.01 compared to the r-Antidote treatment group determined by unpaired two-tailed t test. |
| In vitro | In patch clamp hERG assays, Betrixaban has IC50 of 8.9 μM. The plasma kallikrein IC50 and Ki values for Betrixaban are 6.3 μM and 3.5 μM respectively. Betrixaban (hERG Ki 1.8 μM) exhibits significantly lower hERG activity than all the others (hERG Ki?0.5 μM) |
| In vivo | Dosed at 0.5 mg/kg IV and 2.5 mg/kg PO, Betrixaban has bioavailability of 51.6% in dog; dosed at 0.75 mg/kg IV and 7.5 mg/kg PO, Betrixaban has bioavailability of 58.7% in monkey[1]. Both Betrixaban and Apixa-ban-mediated whole-blood INR increases are similarly reversed by r-Antidote. After i.v. infusion of the three fXa inhibitors (each admin¬istered individually) for 30 min, the total plasma concentrations of rivaroxaban, Betrixaban and apixaban are 1.4±0.4 μM (mean±s.d.), 0.2±0.01 μM and 1.4±0.3 μM, respectively, and the percentages of unbound inhibitor are 2.2%±0.8% (mean±s.d.), 40%±7.2% and 1.5%±0.3%, respectively. After administration of r-Antidote, the total plasma concentrations of the inhibitors increased to 1.9±0.09 μM, 2.0±0.4 μM and 4.2±0.7 μM, respectively, and the percentage of unbound inhibitor declined to 0%, 0.3%±0.1% and 0.05%±0.02%, respectively. Therefore, for each of the three inhibitors, correction of prothrombin time by r-Antidote to near-normal values is associated with a reduction in the free fraction of the inhibitor[2]. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 16.67 mg/mL (36.88 mM), Sonication is recommended.
|
| Keywords | PRT-054021 | Betrixaban | Factor Xa | inhibit | Fxa | PRT 054021 | Inhibitor |
| Inhibitors Related | Edoxaban | Heparin sodium salt | 2-Methoxyphenothiazine | Coumarin |
| Related Compound Libraries | Bioactive Compound Library | EMA Approved Drug Library | Drug Repurposing Compound Library | Inhibitor Library | FDA-Approved Drug Library | Metabolism Compound Library | Bioactive Compounds Library Max |

 United States
United States