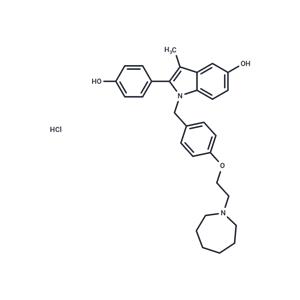
| Name | Bazedoxifene hydrochloride |
| Description | Bazedoxifene hydrochloride (TSE 424 hydrochloride) is an orally active, selective, and potent estrogen receptor modulator (SERM) that crosses the blood-brain barrier and is an inhibitor of IL-6/GP130 protein interactions with high affinity for ERα and ERβ. It has a high affinity for ERα and ERβ and can be used to study postmenopausal osteoporosis and vasodilator-related diseases. |
| Cell Research | For the proliferation assay, cells are plated at 20,000 cells/well in a 24-well plate in DMEM/F12 (50:50) (phenol red-free) with 10% charcoal/dextran-treated FBS and 1 × GlutaMAX-1. After overnight incubation, the medium is aspirated and treatments in DMEM/F12 (50:50) (phenol red-free) with 2% charcoal/dextran-treated FBS and 1 × GlutaMAX-1 are added to the wells. Each plate has a vehicle (baseline proliferation) and treatments. Treatments included 10 pM 17β-estradiol determined to be the EC80 for 17β-estradiol and 17β-estradiol in combination with six concentrations of BZA. Treatments from d 1 are renewed on d 3 and d 6 by aspirating medium from wells and replacing with fresh medium and treatments. On d 7, cells are detached from the plate using trypsin-EDTA and counted using a Multisizer II.(Only for Reference) |
| Kinase Assay | Ligand binding competition experiments: Test compounds are initially solubilized in DMSO and the final concentration of DMSO in the binding assay is ≤ 1%. Eight dilutions of each test compound are used as an unlabelled competitor for [3H]17β-estradiol. Typically, a set of compound dilutions would be tested simultaneously on human, rat and mouse ER-α and ER-β. The results are plotted as measured DPM vs. concentration of test compound. For dose-response curve fitting, a four parameter logistic model on the transformed, weighted data are fit and the IC50 is defined as the concentration of compound decreasing maximum [3H]estradiol binding by 50%. For active compounds, the IC50 is determined at least three times. It should be noted that IC50 values are not direct measures of a ligand's affinity for the receptor. Rather, they can only be compared as relative values, in this case to 17β-estradiol. |
| In vitro | In AsPC-1 cells, Bazedoxifene hydrochloride (at concentrations of 10 μM and 20 μM; treated for 2 hours) is able to inhibit STAT3 phosphorylation induced by IL-6, IL-11, or OSM (each at 50 ng/mL)[2]. |
| In vivo | In 6-week-old female athymic nude mice, Bazedoxifene hydrochloride ( 5 mg/kg ; oral gavage, daily, for 18 days ) was used to Suppressed pancreatic cancer xenograft tumor growth and induced apoptosis in tumor cells[2]. |
| Storage | store at low temperature,keep away from direct sunlight | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | H2O : <1 mg/mL
Ethanol : <1 mg/mL
DMSO : 93 mg/mL (183.4 mM)
|
| Keywords | TSE-424 | TSE424 | TSE 424 | STAT3 | SERM | selective | receptor | protein-protein | modulator | interactions | IL-6/GP130 | Estrogen Receptor/ERR | estrogen | ERβ | ERα | Bazedoxifene HCl | Bazedoxifene |
| Inhibitors Related | Sodium Thiocyanate | Disulfiram | Inosine pranobex | Kaempferol | Tamoxifen | Amlexanox | Mifepristone | Estradiol | Dexamethasone | Melatonin | Diallyl disulfide | Ethisterone |
| Related Compound Libraries | FDA-Approved & Pharmacopeia Drug Library | Bioactive Compound Library | Approved Drug Library | Drug Repurposing Compound Library | Inhibitor Library | FDA-Approved Drug Library | Orally Active Compound Library | Immunology/Inflammation Compound Library | Bioactive Compounds Library Max | Anti-Cancer Compound Library |

 United States
United States