Product Number: A006140
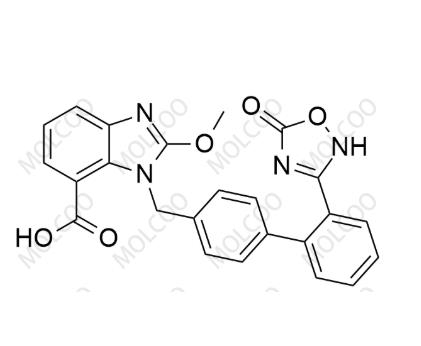
English Name: Azilsartan Impurity 140
English Alias: 2-methoxy-1-((2'-(5-oxo-2,5-dihydro-1,2,4-oxadiazol-3-yl)-[1,1'-biphenyl]-4-yl)methyl)-1H-benzo[d]imidazole-7-carboxylic acid
CAS Number: 147403-35-8
Molecular Formula: C₂₄H₁₈N₄O₅
Molecular Weight: 442.42
As an impurity reference standard for azilsartan, this compound has the following advantages:
Well-defined structure and high stability, enabling precise analysis of by-product formation mechanisms during azilsartan synthesis, such as benzimidazole ring construction and oxadiazole introduction, to optimize processes and reduce impurity generation;
As a reference standard with nitrogen heterocyclic and carboxyl structures, it provides a standard substance for detecting impurities with complex functional groups in drugs, improving the quantitative accuracy of methods like HPLC and LC-MS;
Helps study the impact of heterocyclic structures on drug stability and toxicological properties, providing a scientific basis for impurity control strategies.
Drug Development: Used as an impurity reference standard to identify and quantify Impurity 140 in azilsartan preparations, evaluating the purity of APIs and formulations;
Quality Control: Acting as a standard substance to validate the sensitivity and specificity of detection methods (e.g., HPLC, LC-MS), ensuring the impurity content meets pharmacopoeia and regulatory requirements during production;
Stability Studies: Investigating the degradation behavior of this impurity under light, high temperature, and high humidity conditions to evaluate its impact on azilsartan formulation stability, assisting in determining storage conditions and shelf life.
Azilsartan is an angiotensin II receptor antagonist used for treating hypertension. During its synthesis, if reaction conditions are not properly controlled, such as incomplete cyclization reactions or abnormal substituent introduction, impurities like Azilsartan Impurity 140 with oxadiazole and benzimidazole structures may be generated. Since such impurities may affect drug safety and effectiveness, research on them is a key part of azilsartan quality control and safety assessment.
Current research focuses on:
Detection Method Optimization: Establishing trace detection methods for this impurity using techniques such as ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) and high-resolution mass spectrometry (HRMS), achieving precise analysis with sensitivity reaching the ppb level;
Synthesis Process Improvement: Optimizing the synthesis route by adjusting catalyst types, reaction temperature, and raw material ratios to reduce impurity generation and developing high-purity impurity synthesis processes;
Toxicological Evaluation: Studying the potential toxicity of this impurity through in vitro cytotoxicity experiments and animal models to provide data support for formulating reasonable impurity limit standards;
Crystal Form and Physicochemical Properties: Investigating the crystal form characteristics of this impurity and its impact on the physical stability of drug formulations to improve the azilsartan quality control system.





 China
China