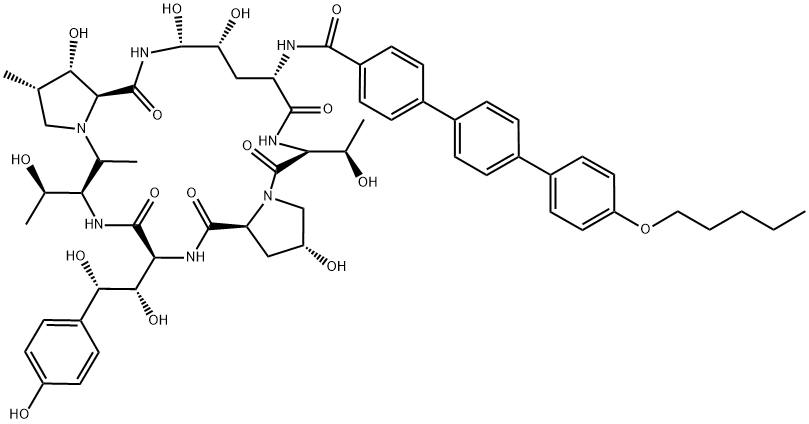
| CAS: | 166663-25-8 |
| MF: | C58H73N7O17 |
| MW: | 1140.24 |
| EINECS: | 658-060-4 |
| Product Categories: | Inhibitors;Antifungals;API |
| Mol File: | 166663-25-8.mol |
 |
|
| Anidulafungin Chemical Properties |
| Melting point | >196°C (subl.) |
| Boiling point | 1477.0±65.0 °C(Predicted) |
| density | 1.47±0.1 g/cm3(Predicted) |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly) |
| pka | 9.86±0.26(Predicted) |
| form | Solid |
| color | White to Pale Beige |
| Anidulafungin Usage And Synthesis |
| Description | Anidulafungin, a semi-synthetic derivative of echinocandin B, has been developed and launched as an intravenous treatment for serious fungal infections, such as candidemia, Candida-derived peritonitis, intra-abdominal abscesses, and esophageal candidiasis. As a non-competitive inhibitor of 1,3-b-D-glucan synthase, which is responsible for the formation of glucan polymers, anidulafungin interferes with the cell wall synthesis of most pathogenic fungi. This mode of action is characteristic of the echinocandin class of antifungals. While the first member of this class, cilofungin, was withdrawn due to toxicity associated with the formulation vehicle, anidulafungin follows the successful introduction of caspofungin and micafungin. Compared to the other echinocandins, anidulafungin appears to be more potent (MIC90 ofr0.25 mg/mL for C.albicans, 0.5 mg/mL for C.glabrata, 1 mg/mL for C. krusel and C.tropicalis, 2mg/mL for C.lusitaniae, and 2 mg/mL for Aspergillus spp) and is devoid of significant drug interactions since it is neither an inhibitor nor substrate of the cytochrome P450 isoenzymes. The emergence of the echinocandins circumvents the concern regarding the rising resistance to the azole and amphotericin B antifungals; no cross-resistance is expected because the echinocandins work at the cell wall rather than the cell membrane. |
| Originator | Eli Lilly (US) |
| Uses | Anidulafungin is a semi-synthetic cyclic lipopeptide belonging to the echinocandin class that was reported in 1995 and commercially developed by Eli Lilly. Anidulafungin inhibits the synthesis of β-(1,3)-D-glucan, an essential component of the cell wall of susceptible fungi and is extensively referenced in the literature with over 400 citations. |
| Uses | nucleoside reverse transcriptase inhibitor (NRTI) for HIV treatment in adults |
| Definition | ChEBI: A semisynthetic echinocandin anti-fungal drug. It is active against Aspergillus and Candida species and is used for the treatment of invasive candidiasis. |
| Brand name | Eraxis (Vicuron). |
| Antimicrobial activity | It is active against Aspergillus spp., Candida spp. and the cyst stage of Pneumocystis jirovecii. Resistance has not yet been reported. |
| Pharmaceutical Applications | A semisynthetic lipopeptide derived from a fermentation product of Aspergillus nidulans. Formulated for intravenous infusion. |
| Pharmacokinetics | Cmax 100 mg 1-h infusion: c. 9 mg/L end infusion
Plasma half-life: 18–27 h
Volume of distribution: 0.6 L/kg
Plasma protein binding: 84%
Blood concentrations increase in proportion to dosage. The steady state is achieved on the first day after a loading dose (twice the daily maintenance dose).
Distribution
Levels in the CSF are negligible.
Metabolism and excretion
Unlike caspofungin and micafungin, anidulafungin is not metabolized by the liver, but undergoes slow non-enzymatic degradation in the blood to a peptide breakdown product which is enzymatically degraded and excreted in the feces and bile. About 30% of a dose is eliminated in the feces, of which less than 10% is unchanged drug. Less than 1% of a dose is excreted in the urine. No dosage adjustment is required in patients with hepatic or renal impairment. Anidulafungin is not cleared by hemodialysis. |
| Clinical Use | Candidemia and certain invasive forms of candidosis
Esophageal candidosis |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock


 Japan
Japan







 Company information
Company information Advantage
Advantage


