Manufacturer Provide High Quality CAS34642-77-8 Amoxicillin Sodium

Introduction
1.Product Name: Amoxicillin Sodium
2.Other name : Amoxycilline Sodium Sterile
3.CAS NO: 34642-77-8
4.Purity: 99%
5.Appearance: White Powder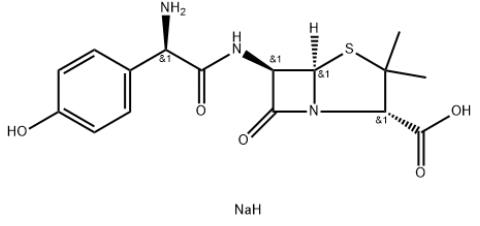
6.Molecular formula: C16H20N3NaO5S
7.Molecular weight: 389.4
8.MeltingPoint :130℃ (dec.)(lit.)
9.Solubility: Highly soluble in water, slightly soluble in anhydrous ethanol, very slightly soluble in acetone.
Amoxicillin sodium is under the trade name Calmoxil. Amoxicillin sodium is well absorbed by oral administration and intramuscular injection, with high bioavailability and rapid elimination. Compared with the amoxicillin sodium long-acting preparation intramuscular injection, the elimination half-life of amoxicillin sodium was significantly prolonged, and there was no significant difference in peak concentration and peak time. Amoxicillin sodium can be administered twice a day, and amoxicillin sodium long-acting preparations can be administered once a day for infection of sensitive bacteria.
Amoxicillin sodium is suitable for patients with the following infections caused by sensitive bacteria who are severely ill and require hospitalization or cannot be taken orally:
1. It is suitable for the treatment of the following infections caused by sensitive bacteria:
1) Upper respiratory tract infections such as otitis media, sinusitis, pharyngitis, tonsillitis, etc.;
2) Acute bronchitis, pneumonia and other lower respiratory tract infections;
3) Urinary and Chemicalbook reproductive tract infections;
4) Skin and soft tissue infections.
2. Applicable to the treatment of acute simple gonorrhea.
3. It can also be used to treat typhoid fever, typhoid fever carriers and leptospirosis.
4. It can still be combined with clarithromycin and lansoprazole to eradicate Helicobacter pylori in the stomach and duodenum and reduce the recurrence rate of peptic ulcer.
| HF160822 | Quantity | 1240kgs |
| Mfg. Date | Aug. 22, 2016 | Package | As required |
| Rep. Date | Aug. 25, 2016 | Exp. Date | Aug. 21, 2018 |
| Items | Standards | Results |
| Appearance | White or off-white powder | Off-white powder |
| Identification | By HPLC | Complies |
| Melting Point | 120ºC~130ºC | 126ºC |
| Loss on Drying | ≤1.00% | 0.21% |
| Heavy Metals | ≤ 10ppm | Complies |
| Sulfated Ash | ≤ 0.10% | 0.03% |
| Chlorine Content | 17.5%~19.5% | 19.3% |
| Arsenic Content | ≤ 0.1ppm | 0.09ppm |
| Lead VContent | ≤ 3.0ppm | 0.50ppm |
| Cadmium Content | ≤ 0.1ppm | 0.10ppm |
| Mercury Content | ≤ 0.1ppm | 0.10ppm |
| Microbiological Test | Coliforms:Negative | Negative |
| Salmonella: Negative | Complies |
| Total plate count: ≤1000 CFU/g | Pass |
| Yeast&Mold: ≤50 CFU/g | Pass |
| Related Substances | Impurity A: ≤0.30% | 0.18% |
| Other unknown impurity: ≤0.10% | 0.07% |
| Total Impurities: ≤0.50% | 0.42% |
| Assay | ≥ 98.0% | 99.50% |
| Reference Standard | USP Standard |


 China
China










