1/5
Ammonium thiocyanate NEW
- Min. Order1KG
- Purity99%
- Cas No1762-95-4
- Supply Ability50000KG/month
- Update time2025-05-16

| Product Name | Ammonium thiocyanate |
| CAS No | 1762-95-4 |
| EC-No | 217-175-6 |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 50000KG/month |
| Release date | 2025/05/16 |
| CAS: | 1762-95-4 |
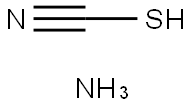
| MF: | CH4N2S |
| MW: | 76.12 |
| EINECS: | 217-175-6 |
| Product Categories: | ACS Grade;Building Blocks;Chemical Synthesis;Essential Chemicals;Inorganic Salts;Organic Building Blocks;Research Essentials;Solutions and Reagents;Sulfur Compounds;Thiocyanates/Isothiocyanates;A - F;By Reference Material;Reference Material Silver nitrate;Salt Solutions;Industrial/Fine Chemicals;straight chain compounds;Inorganics;Volumetric Solutions;Acetals/Ketals/Ortho Esters;Analytical Reagents;Analytical/Chromatography;Oxygen Compounds;Titration;fine chemicals;bc0001 |
| Mol File: | 1762-95-4.mol |
 | |
| Ammonium thiocyanate Chemical Properties |
| Melting point | 152-154 °C (lit.) |
| Boiling point | 262.85°C |
| density | 1.3 |
| vapor pressure | <1 hPa (20 °C) |
| refractive index | 1.5300 (estimate) |
| Fp | 190 °C |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| Specific Gravity | 1.305 |
| color | clear colorless or white |
| Odor | Odorless |
| PH | 4.8-5.8 (50g/l, H2O, 20℃) |
| PH Range | 4.5 - 6.0 |
| Water Solubility | 163 g/100 mL (20 ºC) |
| Sensitive | Light Sensitive & Hygroscopic |
| Merck | 14,561 |
| BRN | 3595135 |
| Exposure limits | NIOSH: IDLH 25 mg/m3 |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents. Forms explosive mixtures with lead nitrate. |
| LogP | 0.58 |
| CAS DataBase Reference | 1762-95-4(CAS DataBase Reference) |
| EPA Substance Registry System | Ammonium thiocyanate (1762-95-4) |
| Ammonium thiocyanate Usage And Synthesis |
| Outline | Ammonium thiocyanate is colorless lustrous monoclinic flaky or columnar crystal with the chemical formula being NH4SCN and the molecular weight of 76.12. At 92 ℃, it is rhombohedral crystal with the melting point of 147 ℃ (decompose at 170 ℃), the relative density of 1.3057 and the refractive index of 1.685. It is easily soluble in water and will have endothermic reaction when being dissolved in water. It is soluble in alcohol, alkali metal hydroxide, acetone, pyridine and liquid sulfur dioxide but insoluble in chloroform. Its solution exhibits red color upon exposure to sunlight.In case of ferric iron, it generates iron thiocyanate of blood red color and can be measured by colorimetric quantification according to the color of standard solution. When being heated to 140 ℃, it can generate Thiourea while it will be decomposed into ammonia, hydrogen sulfide and carbon disulfide at 176 ℃. It is easily to be deliquescent in the air and therefore should be sealed with isolation from acid. Production method: have carbon disulfide and ammonia in a pressurized reactor react for 20 hours at the temperature of 30 ℃ to obtain it; alternatively, have sulfur powder, sodium cyanide and ammonium chloride reacted with water with the temperature being maintained at 110 ℃ to obtain the final product. The main purposes: It can be used as the polymerization catalysts for organic synthesis, dyeing spreading agent, herbicide, analytical reagents such as for determination of mercury, silver as well as trace iron. It can also be used for the production of thiocyanate and thiocyanate complex salt. It also has certain dissolving capability for silver halide. Its concentrated solution has also the ability of dissolving the gelatin. It can also be used as photographic liquid and golden color matching of the printing paper, printing of cotton and steel pickling. |
| Industrial ammonium thiocyanate | The industrial ammonium thiocyanate is one of the purified products of coke oven gas. The pure product is colorless and shiny monoclinic crystal with the formula being NH4SCN and the density being 1.305g/cm3. It is easily deliquescent in air and is soluble in water (upon dissolving industrial ammonium thiocyanate, the production system is highly endothermic). It has a melting point of 149.6 ℃ and decomposition point of 170 ℃. Ammonium thiocyanate is mainly used in dyeing industry and can also be used as chemical reagent or preparation of herbicides. During the purification process of coke oven gas, adopting ammonium polysulfide solution for removing hydrogen cyanide from the oven gas can lead to the industrial ammonium thiocyanate product with the content being more than 92%. The absorption reaction of decyanation of the coke oven gas is: HCN + (NH4) 2Sn → HSCN + (NH4) 2Sn-1 NH3 + HSCN → NH4SCN NH3 + HCN → NH4CN NH4CN + (NH4) 2Sn → NH4SCN + (NH4) 2Sn-1 The ammonia involved in the absorption reaction comes from the coke oven gas itself, so the decyanation device must be in front of the procedure of ammonia recovery of coke oven gas. After the coke oven gas is subjecting to removal of tar mist and naphthalene through the electric tar precipitator, we should further use ammonium polysulfide solution for removing the hydrogen cyanide gas in the oven gas for generating ammonium thiocyanate. The absorbing liquid is constantly circulating and washing the coal gas to increase the concentration of the ammonium thiocyanate contained in the absorption solution while continuously removing a portion of the absorbing liquid for adding sulfur for regeneration. The regeneration reaction formula is (NH4) 2Sn-1 + S → (NH4) 2Sn. When the content of the ammonium thiocyanate of the absorption liquid has reached 250g/L, you can take it out for sending for processing, after the process of evaporation and crystallization, you can produce the industrial product of ammonium thiocyanate. |
| thiocyanic acid | Thiocyanate has two isomers. The first one is thiocyanate H-S-C≡N, the other one is isothiocyanate H-N-C = S. Free acid has not been separated yet. Most of thiocyanate salt can be dissolved in water. SCN-ion is a good ligand. SCN-ion has a special and sensitive reaction of forming various kinds of red complex with Fe3 +. Therefore, potassium thiocyanate or ammonium thiocyanate can often be used as the reagent for analyzing Fe3+. It is colorless, volatile liquid with slightly toxic. It has a melting point of-110 ℃ and is stable at 0 ℃. At room temperature, it can be subject to rapid decomposition at room temperature. It is highly water-soluble and its aqueous solution exhibits strongly acidic. Its thermal solution or concentrated solution is both easy to be broken and easy to be polymerized. At-90 ~-85 ℃, it can be polymerized into white crystals. In vacuum condition, it can be heated to form a pale yellow sulfur cyanuric acid which can be dissolved in diethyl ether. Then it is easily to be broken down into thiocyanate. It can be obtained through the reaction between potassium thiocyanate and potassium hydrogen sulfate or through putting the ammonium thiocyanate aqueous solution through H-cation exchange resin system. In laboratory, its salt can be used testing of iron and quantitative determination of silver reagents. The above information is edited by the Chemicalbook of Dai Xiongfeng. |
| Potassium thiocyanate | Potassium thiocyanate is also known as "sulfur potassium cyanide." Its chemical formula is KSCN with a molecular weight of 97.18. It is colorless rhombic system crystal and has deliquescence. It is toxic and can irritate the skin. It has a melting point of 173.2 ℃ and the relative density of 1.88614. When the melt is cooled, it will change into brown, green, blue and white color. It is easily soluble in water with the aqueous solution being neutral. It is soluble in acetone, ethanol and liquid ammonia. It can subject to decomposition at 500 ℃. Method: potassium cyanide and sulfur powder were co-heated; alternatively, have ammonium thiocyanate and potassium hydroxide react to obtain it. Purposes: for the production of mustard oil, thio-urea, drugs; it can be used for the printing and dyeing industry, photography, and used as a refrigerant and analysis reagents. |
| Solubility in water (g / 100ml) | The amount (gram) dissolved per 100 ml of water at different temperatures (℃): 120g/0 ℃; 144g/10 ℃; 170g/20 ℃; 208g/30 ℃; 234g/40 ℃;346g/60 ℃. |
| Chemical Properties | It is colorless monoclinic flake or columnar crystals. It is shiny. It is easily soluble in water, ethanol, ammonia, acetone, pyridine and liquid sulfur dioxide. |
| Uses | It can be used as dyes and the polymerization catalysts for organic synthesis. It can also be used as pesticides of weeding and defoliants as well as antibiotics separation. It can also be used as the raw material for the manufacturing of cyanide and thiourea. It can be used as the auxiliary materials of the manufacturing of hydrogen peroxide. It can also be also used in dyes, organic synthesis, pesticides, pharmaceuticals, etc. It can also be used for zinc coating, dyeing spreading agent and electroplating additives. It can be used as reagents for the analysis as well as for the separation of antibiotics. It can be used for the verification and determination of trace iron and determination of silver and mercury. It can also be used as polymerization catalyst, for the separation of antibiotics, for pesticides content analysis, water analysis, and preparation of thiocyanate standard solution. |
| Production method | Carbon disulfide method: mix carbon disulfide and a slight excess amount of ammonia with water for reaction of about 20 h at a pressure of 5.88 × 105Pa and at a temperature of 100 ℃ for generating ammonium thiocyanate. The reaction solution was subject to evaporation under reduced pressure for removing the hydrogen sulfide. At a solution temperature of 105 ℃, use ammonium sulfide to remove iron and heavy metals, filter and have the filtrate concentrated under reduced pressure for cooling crystallization in the mold, and then have isolation through centrifugation, dying to obtain ammonium thiocyanate. CS2 + 3NH3 → NH4SCN + NH4HS NH4HS → NH3 ↑ + H2S ↑ Sulfur method: put proper amount of water and sulfur powder into the reactor and stir into a paste like status; add slowly graded solid sodium cyanide for reaction to obtain sulfur sodium cyanide at a temperature of about 110 ℃. Then further add solid ammonium chloride for the reaction to obtain sulfur ammonium cyanate. The reaction mixture was further added into barium thiocyanate; clear the impurities for clarification; the supernatant was evaporated under reduced pressure with concentration to precipitate out the sodium chloride; then filter, cool and crystallize, separate and dry to obtain ammonium thiocyanate. NaCN + S → NaSCN NaSCN + NH4Cl → NaCl + NH4SCN |
| Category | toxic substances |
| Toxicity grading | highly toxic |
Product picture

Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock

Company Profile Introduction
-







