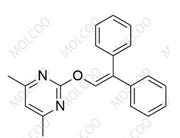
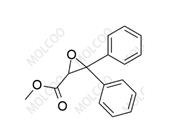
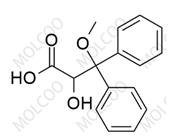
Ambrisentan Impurity 178306-47-3
Ambrisentan Impurity Reference Standard
Utilized in pharmaceutical R&D, quality control, and regulatory submissions as an analytical standard to detect and quantify impurities in drug substances, ensuring drug safety and efficacy.
High Purity Assurance:Synthesized and purified under rigorous protocols to ensure single-component impurities.
Regulatory Compliance:Adheres to USP, EP, BP pharmacopeia standards, supporting global regulatory filings.
Stability:Stable for up to 3 years when stored at -20°C.
Storage:Store at -20°C, protected from light and moisture.
Shipping:Transported under cryogenic conditions (dry ice or cold chain) to maintain stability.
application area :
Impurity profiling in new drug development
Process optimization and quality control in drug manufacturing
Generic drug bioequivalence studies




 China
China