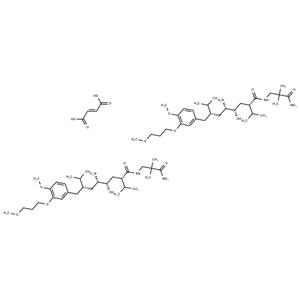
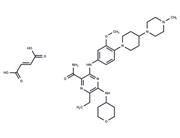
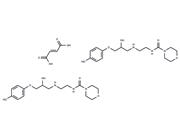
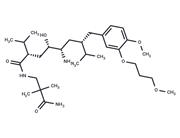
| Name | Aliskiren hemifumarate |
| Description | Aliskiren hemifumarate (CGP60536B) is an orally active nonpeptide renin inhibitor with antihypertensive activity. |
| Kinase Assay | Radioligand Binding Assay: Human CB1 and CB2 stably transfect HEK 293 cells and cell membrane is purified. 0.2-8 μg of the purified membrane is incubated with 0.75 nM [3H] CP55,940 and Rimonabant in the incubation buffer (50 mM Tris-HCl, 5 mM MgCl2, 1 mM EDTA, 0.3%BSA, pH 7.4). The non-specific binding is defined in the presence of 1 μM of CP55,940. The reactions are incubated for one and a half hours at 30 °C in Multiscreen. The reactions are terminated by manifold filtration and washed four times with ice-cold wash buffer (50 mM Tris, pH 7.4, 0.25% BSA).The radioactivity bound to the filters is measured by Topcount. The IC50 is determined as the concentration of Rimonabant required to inhibit 50% of the binding of [3H] CP55,940 and calculated by non-linear regression. |
| In vitro | Aliskiren hemifumarate appears to bind to both the hydrophobic S1/S3-binding pocket and to a large, distinct subpocket that extends from the S3-binding site towards the hydrophobic core of renin. Oral bioavailability of Aliskiren hemifumarate is 2.4% in rats, 16% in marmosets and about 2.5% in humans. [2] |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 93 mg/mL (76.3 mM)
H2O : 92 mg/mL (75.4 mM)
Ethanol : 93 mg/mL (76.3 mM)
|
| Keywords | Aliskiren hemifumarate | Aliskiren | SPP 100 hemifumarate | CGP60536B hemifumarate | cardiovascular diseases | SPP-100 | colon carcinoma | cachexia | cancer | Autophagy | CGP 60536 hemifumarate | Aliskiren Hemifumarate | Renin | inhibit | Hypertension | C26 | SPP100 | CGP60536 | CGP-60536 | Inhibitor |
| Inhibitors Related | Stavudine | Sodium 4-phenylbutyrate | Hydroxychloroquine | Guanidine hydrochloride | Taurine | Curcumin | Paeonol | Naringin | Gefitinib | Captopril |
| Related Compound Libraries | Target-Focused Phenotypic Screening Library | Bioactive Compound Library | Drug-induced Liver Injury (DILI) Compound Library | Anti-Cancer Clinical Compound Library | Drug Repurposing Compound Library | Anti-Cancer Approved Drug Library | FDA-Approved Drug Library | Bioactive Compounds Library Max | Anti-Metabolism Disease Compound Library | Anti-Cancer Drug Library |

 United States
United States