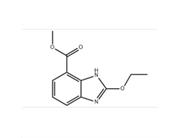
Abhisili is an oral inhibitor of cyclin dependent kinase CDK4/6. In HR+, HER2 breast cancer cells, CDK4 and CDK6 can promote the phosphorylation of retinoblastoma protein (Rb), and promote cell cycle progression and cell proliferation. Abemecilib can inhibit the phosphorylation of Rb and block the progression of cells from G1 phase to S phase of cell cycle, leading to cell aging and apoptosis. On December 29, 2020, Eli Lilly Pharmaceutical announced its Chemicalbook anti-tumor new drug alternative ® (Abbesili Tablets) has been approved by the National Drug Administration (NMPA) for use in locally advanced or metastatic breast cancer with hormone receptor positive (HR+) and human epidermal growth factor receptor negative (HER2 -): (1) It is used in combination with aromatase inhibitors as the initial endocrine therapy for postmenopausal women. (2) It is used in combination with fluvastatin in patients with disease progression after receiving endocrine therapy in the past. Thus, Abemecilib became the second CDK4/6 inhibitor to be listed in China after Piperacili.

 China
China