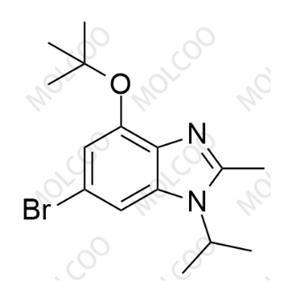
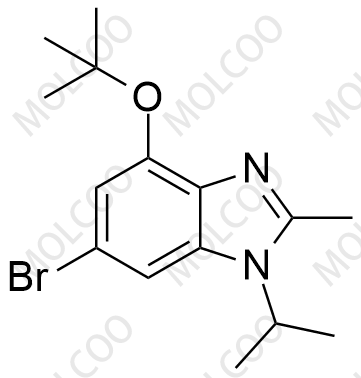
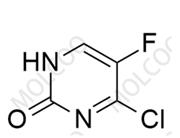
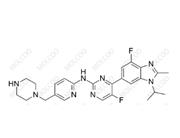
Abemaciclib Impurity 2177297-46-8
Abemaciclib Impurity Reference Standards
Abemaciclib is an oral selective inhibitor of cyclin-dependent kinases 4 and 6 (CDK4/6), primarily used for the treatment of hormone receptor-positive, human epidermal growth factor receptor 2-negative (HR+/HER2-) locally advanced or metastatic breast cancer, as well as adjuvant therapy for early breast cancer. To ensure the quality of Abemaciclib, researchers and pharmaceutical companies require high-quality impurity reference standards for quality control and research and development.
We offer a range of Abemaciclib impurity reference standards, including impurities such as Abemaciclib Impurity C and Abemaciclib Impurity D. These impurity reference standards have undergone rigorous purification and identification processes, with purities exceeding 95%, making them suitable for various applications such as project approval, drug development, and quality control.
Our Abemaciclib impurity reference standards feature the following characteristics:
High Purity: All impurity reference standards have purities above 95%, ensuring the accuracy of results.
Multiple Specifications: We offer various packaging specifications, including 10mg, 25mg, 50mg, and 100mg, to meet different needs.
Strict Quality Control: Each batch of impurity reference standards undergoes rigorous quality control, including the provision of Certificates of Analysis (COA), Nuclear Magnetic Resonance (NMR), High-Performance Liquid Chromatography (HPLC), and Mass Spectrometry (MS) spectra, to ensure product quality.
Welcome inquiries and orders from researchers and pharmaceutical companies!


 China
China