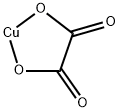
Chemical Properties
Cupric oxalate is a bluish-white, odorless powder.
Uses
As catalyst for organic reactions; as stabilizer for acetylated polyformaldehyde; in anticaries compositions; in seed treatments to repel birds and rodents.
General Description
Odorless bluish-white solid. Denser than water and insoluble in water. Hence sinks in water. Used as a catalysts for organic reactions.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Cupric oxalate dissolves in aqueous ammonia and reacts as an acid to neutralize other bases as well. Can serve as a reducing agent in reactions that generate carbon dioxide.
Hazard
Toxic by ingestion; tissue irritant.
Health Hazard
Inhalation causes irritation of nose and throat. Ingestion of very large amounts may produce symptoms of oxalate poisoning; watch for edema of the glottis and delayed constriction of esophagus. Contact with eyes causes irritation.
Fire Hazard
Special Hazards of Combustion Products: Toxic carbon monoxide gas may form in fire.
Potential Exposure
Used as a catalyst for organic reactions and in seed treatment as a repellent for birds and rodents.
Shipping
UN2775, Copper based pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Explosive materials are formed on contact with acetylene gas, ammonia, caustic solutions; sodium hypobromite, nitromethane. Slight heating can cause a weak explosion. Cupric oxalate dissolves in aqueous ammonia and reacts as an acid to neutralize other bases as well. Can serve as a reducing agent in reactions that generate carbon dioxide. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.

 China
China