Uses
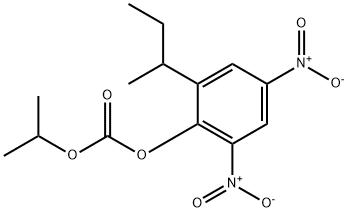
Dinobuton is a non-systemic fungicide, active against powdery mildews in apples, cotton and vegetables.
Metabolic pathway
Dinobuton is metabolised in plants and animals via common metabolic pathways. The primary reaction is hydrolytic cleavage of the carbonate-dinitrophenyl linkage to yield dinoseb. Further reduction of the nitro-groups of dinoseb yields the corresponding monoamino and diamino analogues. Acetylation and deamination via hydroxylation/ elimination, and oxidation of the sec-butyl moiety also occurred. N- and O-Conjugation as glucosides and glucuronides occurred in both plants and animals. The metabolic pathways of dinobuton are presented in Scheme 1.
Degradation
There is limited information on the hydrolytic stability of dinobuton (1). Marchenko and Vakulenko (1983) reported that the hydrolysis DT50 values of dinobuton in pH 2, 7 and 11 buffer solutions at 22 °C were 15 days, 17 days and 4 hours, respectively. In vitro studies showed the rapid hydrolysis of dinobuton to dinoseb [ 2-sec-butyl-4,6-dinitrophenol (2)] by tissue homogenates of spider mites and animal tissues. Once generated, dinoseb was stable to hydrolytic and photolytic degradation under acidic condition but was readily degraded under alkaline conditions (Brestkin et al., 1978; Molnar, 1935). Uncharacterised complex polar materials were reported as photodecomposition products of dinoseb (Matsuo and Casida, 1970). Grechko et al. (1986) reported that the DTW for dinobuton in aqueous solution under UV light irradiation (365 nm) was <30 min.

 China
China