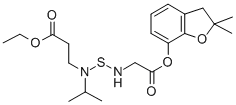
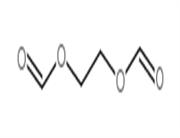
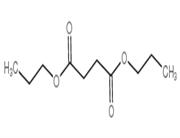
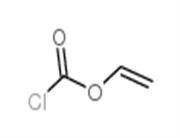
Chemical Properties
Benfuracarb is a thick liquid.
Uses
Benfuracarb is a benzofuranyl methylcarbamate based insecticide used to control aphids, springtails and other pests usually on beet crops.
Uses
Benfuracarb is a contact and ingested insecticide. It is used to control insect pests in citrus, maize, rice, sugar beet and vegetables. It is active against Chrysomelidae, Elateridae, Aphididae, Lissorhoptrus oryzophilus and Plutella xylostella.
Potential Exposure
A benzofuranyl methylcarbamate insecticide, nematicide. Not registered for use in the United States.
Metabolic pathway
When 14C-benfuracarb is applied topically to houseflies, the houseflies metabolize benfuracarb easily to form carbofuran which in turn is oxidized at the 3-position of the ring and N-methyl group, resulting in further conjugates of the metabolites. Major metabolites are carbofuran, 3-hydroxycarbofuran, N- hydroxymethylcarbofuran, 3-ketocarbofuran, 2,3- dihydro-2,2-dimethyl-3-hydroxybenzofuran-6-ol, 3- hydroxy-N-hydroxymethylcarbofuran, and 3-keto-N- hydroxymethylcarbofuran.
Shipping
UN 2992 Carbamate pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1—Poisonous materials.
Degradation
Benfuracarb is stable in neutral and weakly basic media but unstable in strongly acidic or basic conditions. It is degraded by sunlight (PM). A methanolic solution of unlabelled benfuracarb was coated on a glass plate or applied to soil on a plate and irradiated with a high pressure Hg lamp (125 W). Details not given were the emission spectrum of the lamp, the experimental sample temperatures and the irradiation periods. After irradiation, samples were analysed by TLC methods. The methanol solution turned a deep brown on irradiation and four major and three minor products were formed (see Scheme 1). The major products were a cleavage product (2), the phenol (5) and carbofuran (6). Minor amounts of the dimeric compounds 3 and 4 were detected. On soil, three photoproducts were the phenol (5), carbofuran (6) and the cleavage product (7). On a glass surface four products were the phenol (5), carbofuran (6) and the cleavage products 2 and 7. No products of oxidation were reported (Dureja et al., 1990).
Incompatibilities
Carbamates are incompatible with reducing agents, strong acids, oxidizing acids, peroxides, and bases. Contact with active metals or nitrides cause the release of flammable, and potentially explosive, hydrogen gas. May react violently with bromine, ketones. Incompatible with azo dyes, caustics, ammonia, amines, boranes, hydrazines, strong oxidizers.
Waste Disposal
Do not discharge into drains or sewers. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved landfill. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Incineration with effluent gas scrubbing is recommended. Containers must be disposed of properly by following package lab el directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.

 China
China