Uses
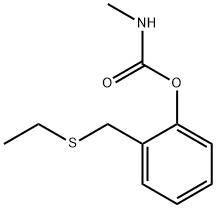
Ethiofencarb is a systemic insecticide with contact and stomach action. It is used to control aphids on fruit, vegetables, ornamentals and sugar beet.
Metabolic pathway
Ethiofencarb is metabolised by rapid oxidation at sulfur, hydrolysis of the carbamate group to give phenols, hydroxylation of the N-methyl moiety and conjugation.
Degradation
Ethiofencarb is stable in neutral and acidic but is hydrolysed under basic conditions. DT50 values at pH 7 and 11.4 (37 °C) were 450 hours and 5 minutes, respectively. The kinetics of hydrolysis of ethiofencarb in pure water and in aqueous solutions at pH 2,6,9,12 and at temperatures in the range 4-50°C were studied. No acid hydrolysis was observed. Ethiofencarb was rapidly hydrolysed at pH 9 and 12. Ethiofencarb in pure water at room temperature reached an equilibrium with 80% remaining undegraded (Sanz-Asensio et al., 1997).
Photodegradation of aqueous solutions in sunlight is rapid (PM). The oxidative photodegradation of ethofencarb was studied in aqueous acetonitrile using anthraquinone to mimic natural photosensitisers. Solutions were irradiated with a Hg lamp (400 W) for 48 minutes. The emission spectrum of the lamp was not described. Reaction products were identified by GC-MS methods. The main products were 2- hydroxybenzaldehyde (2) and 3-methylbenzo[e-1,3]oxazine-2,4-dione(3) (see Scheme 1). Products resulted from photocleavage of the CH,-S bond and/or the C-O bond followed by hydrogen atom abstraction and photo-oxidation. An electron-acceptor photosensitiser may increase rates of photodegradation (Galadi and Julliard, 1996). Solutions of ethiofencarb in cyclohexane, cyclohexene or isopropanol were irradiated with a high pressure Hg lamp (cut-off filter <280 nm) or natural sunlight (Germany, May-July). Analysis was by HPLC with diode-array detection, NMR, IR and MS. Half-lives of photodegradation were in the range 20 minutes to more than 20 hours. The predominant reaction (Scheme 1) was photo-oxidation of ethiofencarb to its sulfoxide (4). The cyclic dione (3) was a product of oxidation at the benzylic position. Ethiofencarb was photo-oxidised in cyclohexane to the sulfoxide (4) and the sulfone (5) and their corresponding phenols (8 and 9), the latter being a minor product. Subsequently the cyclised dione (3) was formed. In isopropanol, reaction with solvent gave addition products (6 and 7) and an unusual bis-diethylthio compound (10) (Kopf and Schwack, 1995).

 China
China