Chemical Properties
Colorless to yellowish, flammable liquid. Aromatic odor.
Uses
Selective herbicide.
General Description
Colorless to yellow liquid with aromatic odor. Non corrosive. Used as an herbicide.
Air & Water Reactions
Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids.
Reactivity Profile
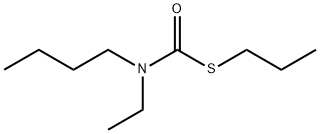
PEBULATE is a thiocarbamate. Flammable gases are generated by the combination of thiocarbamates and dithiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates and dithiocarbamates are incompatible with acids, peroxides, and acid halides.
Potential Exposure
Pebulate is a thiocarbamate herbicide used for pre-emergence control of germinating seeds of broadleaf and grassy weeds in sugar beets, tobacco, and tomatoes. There are no registered residential uses of pebulate
Shipping
Do not discharge into drains or sewers. Dispose of waste material as hazardous waste using a licensed disposal contractor to an approved landfill. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. If allowed, incineration with effluent gas scrubbing (carbon dioxide may be released) is recommended. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Noncombustible containers should be crushed and buried under more than 40 cm of soil. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Incompatibilities
Thiocarbamate esters are combustible. They react violently with powerful oxidizers such as calcium hypochlorite. Poisonous gases are generated by the thermal decomposition of thiocarbamate compounds, including carbon disulfide, oxides of sulfur, oxides of nitrogen, hydrogen sulfide, ammonia, and methylamine.Thio and dithiocarbamates slowly decompose in aqueous solution to form carbon disulfide and methylamine or other amines. Such decompositions are accelerated by acids. Flammable gases are generated by the combination of thiocarbamates with aldehydes, nitrides, and hydrides. Thiocarbamates are incompatible with acids, peroxides, and acid halides.

 China
China