Indications
Thiacetazone is active against many strains of M. tuberculosis. It is not marketed in the United States. However, because of its low cost, it is used as a first-line agent in East Africa, especially in combination with compounds such as isoniazid. The most common side effects of thiacetazone include GI intolerance and development of rashes. It causes significant ototoxicity, especially when coadministered with streptomycin. Lifethreatening hypersensitivity reactions, such as hepatitis, transient marrow aplastic syndromes, neutropenia, and thrombocytopenia, have been reported.
Pharmaceutical Applications
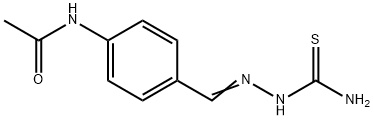
Thioacetazone (USAN amithiozone) is a synthetic compound discovered during initial work on the sulfonamides, to which it is structurally related. It is only slightly soluble in water. It is a weak bacteristatic drug, with frequent serious side effects, particularly in HIV-positive persons to whom it must never knowingly be given. On the advice of the WHO it no longer has a place in the treatment of tuberculosis, except as a last resort in cases of extreme drug resistance.
In-vitro MICs vary considerably according to the medium used and bear little relation to in-vivo efficacy. Many strains of M. tuberculosis isolated in East Africa, India and Hong Kong are naturally more resistant than strains from Europe. Acquired resistance, as a result of monotherapy, is prevalent in the developing countries.
Thioacetazone is well absorbed, achieving a plasma concentration of 1–4 mg/L 2–4 h after a 100 mg oral dose. The plasma half-life is 8–12 h. Little is known about the distribution of the drug. Several metabolites are described. About 20% is eliminated in the urine; the fate of the remainder is unknown. Rashes are common, occurring in 2–4% of patients in Africa but much more frequently in those of Chinese ethnic origin. More severe skin reactions, exfoliative dermatitis and Stevens–Johnson syndrome occur in less than 0.5% of HIV-negative patients, but there is a 10-fold increase of these reactions in HIV-positive patients, proving fatal in up to 3% of such patients. Other common side effects include gastrointestinal reactions, vertigo and conjunctivitis. Less common reactions include hepatitis, erythema multiforme, hemolytic anemia and, rarely, agranulocytosis. Prolonged therapy may rarely lead to hypertrichosis, gynecomastia and osteoporosis. It is very rarely used, but may occasionally be considered (with other antituberculosis drugs) in extremely drug resistant tuberculosis.

 China
China